Continuous Manufacturing and Granulation
The pharmaceutical formulations manufacturing is beginning to concentrate on continuous manufacturing and granulation is one of the key processes that requires to be continuous. Not only does continuous granulation allow for lower costs, but also very efficient and effective manufacturing operations, with a very low space and energy requirement.
With very few new and innovative drugs being approved or in the pipeline, generic manufacturers have a very high advantage when they can make their manufacturing process more cost effective.
A lot of the leading pharmaceutical formulations manufacturers have already started looking at larger / continuous productions processes, and are in active discussions with machine manufacturing companies to come up with such solutions. A large part of the challenge to make the granulation processes continuous is the ability to make it GMP and also the ability to prove a total clean state, once cleaning is completed. Due to above challenges, companies are looking at re-registering their processes by alternative methods like:
Direct Compression
While this method is good for companies that have limited manufacturing capabilities and almost no expertise in granulation, it has its own challenges too. It works with a very few products and materials handling is a key step as well. With direct compression, it is imperative to make sure that segregation is avoided and product consistency is maintained. When it comes to costs, this also becomes an expensive method, as inputs are more expensive and difficult to get to the required standards.
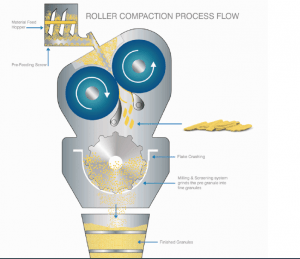
Dry Granulation
Dry Granulation inherently happens to be a continuous granulation method and is around for a number of years. The process requires well designed and engineered equipment that can consistently maintain process parameters and ensure good productivity. There is also a need to have closed and integrated solutions to ensure high yield.
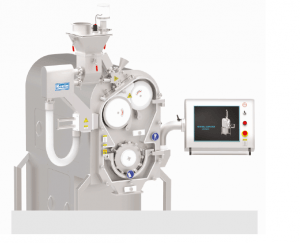
Dry granulation using Roller compactors is the most preferred method today for granulation in a continuous way. Since this is a continuous process, it is easier to achieve Larger Batch sizes and can help lower the cost of manufacturing substantially. Most roller compactors are compact and have a very small foot print, thereby allowing for a smaller GMP area. Closed and integrated operations can be achieved easily compared to other methods of manufacturing without compromising on process.
KEVIN Roller Compaction System offers the following solid benefits, when compared to similar output wet granulation system:
Low investment and low capital expenditure. 
Low space requirement in a pharmaceuticals facility, which generally requires higher costs to maintain environment and GMP.
Low requirement of energy and most importantly no requirement of steam or compressed air.
Low labour cost, as the equipment is small and requires no manual intervention once the process is started.
Reduction in process times thereby increasing plant output/yields.
Saves resources such as Quality Assurances, Validation and Engineering personnel.
Ability to handle large amount of materials in a short period of time.
Ability to handle very small amounts of material.
Close loop controlled and advanced solid screw feeding system for materials in the compaction zone.
Vacuum degeneration system.
Stable and optimized roller design and parameter controls to provide longer dwell times.
Inline oscillating granulator, built in to the compactors for a continuous output of desired granules sizes.
Computer controlled and recipe control for precise control.
Design suitable for dust containment.
Clear separation of process and mechanical area.
SCADA system with 21 CFR part 11 compliance.
Provision for separation of process and technical area.
All motors are connected with gearbox through direct couple and control individual variable drive.
In built granulator for particle size reduction.
Total cycle in close system with visual glass.
User friendly operating screen (HMI).
Flameproof model available for hazardous products.
Various design of rollers for critical products.
This article is contributed by Dr. Parag Shah, Sr General Manager – International Sales and Business Development, Kevin Process Technologies.
