
In recent years, formulations based on pellets have been the trend. New technologies make it possible to circumvent property rights for active ingredients and are therefore very popular with pharmaceutical customers. But which technologies are the most important?
Klaus Möller,
Head of Business Development, Glatt Process Technology Pharma, discusses
In recent years, formulations based on pellets have been the trend. New technologies make it possible to circumvent property rights for active ingredients and are therefore very popular with pharmaceutical customers. But which technologies are the most important?
Klaus Möller,
Head of Business Development, Glatt Process Technology Pharma, discusses.
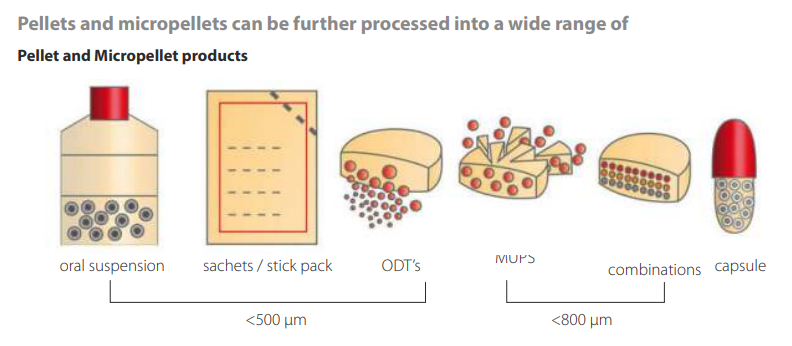
Pellets are the jack-of-all-trades of solid dosage forms. Positioned somewhere between powder and granulate, they make bitter medicine more palatable and can even awaken a child’s instinct to play when the dosage forms are imaginative enough. One well- known example is the Xstraw, a plastic tube shaped like a drinking straw which is filled with pellets of active ingredient, through which children or elderly people can take in the medicine with water. Pellets in tablets are also making a splash – hybrids which combine all the advantages of both dosage forms.
The pioneers in the development of these formulations, known as Multiple Unit Pellet Systems (or MUPS for short), was Astra Zeneca in 1999. Their move to embed the proton pump inhibitor Omeprazole in micropellets and then compress these pellets into immediate release tablets was an award- winning one at the time. The development of MUPS and Xstraw symbolizes the impetus pellets have fueled in recent years. Klaus N. Möller, Head of Business Development at Glatt in Binzen /Germany, explains: “New excipients, coating materials and sophisticated processes allow us to extend the patent protection period and to make the dosage form more attractive.”
The number of patents registered yearly for pellet- based formulations has increased exponentially and is set to continue. According to research performed by IMS Health, the market for OSD (Oral Solid Dosage Forms) is growing by 6 to 8 percent every year. The number of drugs approved by the FDA also reflects this trend: in 2015, more than half were solid products.
Pellets, as defined by pharmacy guru Prof. Peter Kleinbudde are “an isometric agglomerate of powder particles in an approximate spherical or cylindrical form”, and are a task for perfectionists. The smoother and rounder the pellets, the better they are at fulfilling their purpose. The equipment manufacturer Glatt and their specialists from Pharmaceutical Services have been actively pursuing the subject for years.
There are two fundamental ways of making active ingredient pellets: direct pelletization, in which the powdered active ingredient and excipient combine in a matrix, and active ingredient layering, in which uses side spray or Wurster technology to apply the active ingredient to a starter core of sugar or microcrystalline cellulose.
A Case for the Specialists
One interesting process variant for matrix pellets is the extrusion of wet granulate in a basket extruder and subsequent rounding in a spheronizer. Möller elucidates: “Continuous wet granulation, followed by extrusion, spheronization and drying now make it possible to perform continuous processes”. Active ingredient pellets made like this can then be covered with a functional coating, be continuously mixed with excipients and be directly compressed into a MUPS tablet. The challenge is to avoid separation of the ingredients and destruction of the tablets during pressing.
 Glatt, whose portfolio comprises all granulation and pellet manufacturing techniques, has spent recent years developing additional ways of “fine tuning” the pellet process and has opened up a range of new, interesting possibilities for the lifecycle management of active ingredients.
Glatt, whose portfolio comprises all granulation and pellet manufacturing techniques, has spent recent years developing additional ways of “fine tuning” the pellet process and has opened up a range of new, interesting possibilities for the lifecycle management of active ingredients.
Overview of the Various Ways of Making Pellets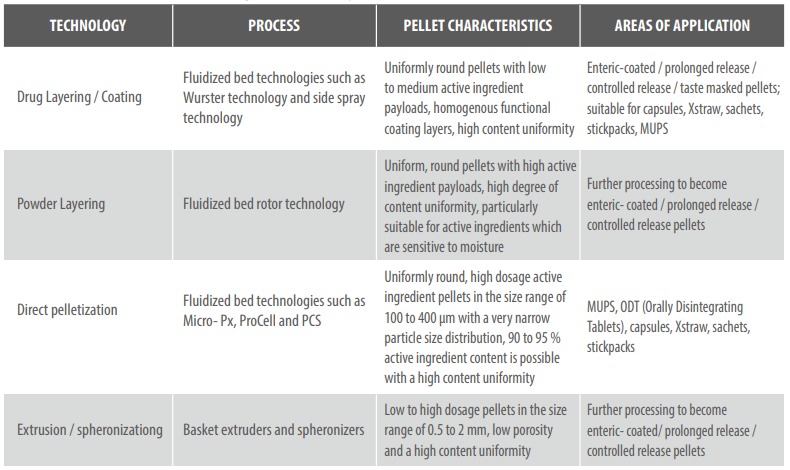
Applying the Final Touches
But what differentiates the manufacturing of granulates from the manufacturing of pellets? From a pharmaceutical point of view, both processes are closely related and are only separated by the form of the particle, since the ideal shape for pellets is a sphere. There are also definite commonalities in procedure.
As Möller explains: “The fluidized bed can be used for both granulation and pelletization. This is why we configure fluidized bed machines on request to be multipurpose installations which then allow the continuous manufacturing of pellets. The individual process modules for direct pelletization with rotor technology, for layering active ingredient and for pellet coating with Wurster technology or the simple drying of wet granulates can be added as necessary.”
 Wurster technology has been used in practice for many years: it is a fluidized bed technique in which starter cores or active ingredient pellets are sprayed with a insists. Möller says: “This method is robust and, because the process is so stable, it’s generally the most popular way to process pellets.”
Wurster technology has been used in practice for many years: it is a fluidized bed technique in which starter cores or active ingredient pellets are sprayed with a insists. Möller says: “This method is robust and, because the process is so stable, it’s generally the most popular way to process pellets.”
Depending on the composition of the tablets, processing can last anywhere between eight and ten hours. The knack is knowing how to optimize the efficiency and times of the production process. Additionally, Möller recommends timely expert assistance during the development of the pellet formulation and the production process: “Right from the beginning, it will help to avoid mistakes and to keep an eye on process times and manufacturing costs.”
MicropelIets and More
Glatt’s development team demonstrated how to refine an established process with the fluidized bed agglomeration technique known as MicroPx. The trick is to use the Conti process, which was conceived in Pharmaceutical Services’ laboratories in Binzen: first, the active ingredient/excipient liquid is spray-dried to a very fine product dust in a fluidized bed and agglomerated into tiny primary particles. The micropellets then build up, layer by layer, until the desired size is reached.
The heart of this technology is a zigzag classifier which continuously ejects particles of sufficient size from the process, while simultaneously allowing smaller particles to reenter the process chamber where they continue to grow. Möller explains that the result of this method is high dosage active ingredient pellets in the size range of 100 to 400 μm with a narrow particle size distribution and content uniformity of a consistent 90 to 95 percent.
This means that one significant limitation of former times is now no longer an issue: for many years, the volume of a pellet-filled capsule was larger – and therefore much harder to swallow – than the equivalent tablet with the same dose and composition. The use of microencapsulation, which changes bitter-tasting active ingredients into tasteless microparticles, means the taste is much improved now, too.
Micropellets can be also pressed into tablets or MUPS tablets which already begin disintegration in the mouth. But the reason pharmaceutical companies find the MicroPx process so exciting is that it makes completely new formulations possible and therefore allows the legal circumvention of property rights. The technology experts have long known the secret to the perfect pellet, too, an answer provided by Complex Perfect Spheres Technology (CPS). CPS is a souped-up rotor process for fluidized bed machines that uses direct pelletization to yield functionalized pellets and micropellets which are perfectly round and smooth.
 Unlike classic rotor technology, the modified technique uses a tapered rotating disc which allows the movement of particles to be directed and pelletization to be performed to a defined endpoint. The results are perfectly spherical pellets of exactly defined sizes of between 100 and 1500 μm and extremely narrow size distribution. This is how Glatt’s own Cellets of microcrystalline cellulose are created, which are used as starter cores for pellets and in the Wurster process, for example – thus completing the formulation cycle.
Unlike classic rotor technology, the modified technique uses a tapered rotating disc which allows the movement of particles to be directed and pelletization to be performed to a defined endpoint. The results are perfectly spherical pellets of exactly defined sizes of between 100 and 1500 μm and extremely narrow size distribution. This is how Glatt’s own Cellets of microcrystalline cellulose are created, which are used as starter cores for pellets and in the Wurster process, for example – thus completing the formulation cycle.
‘A TECHNICAL PLAYGROUND FOR THE PHARMACEUTICAL INDUSTRY’

A word from Klaus Möller, Head of Business Development, Glatt Process Technology Pharma.
The new Innovation Center in Binzen was inaugurated last Fall. What can you offer customers there?
Klaus Möller: We offer the customers a technical playground of 7000 m2 with state-of-the art machine technology. The customer can perform batch and continuous processes in a non-GMP environment. The highly flexible machines can be configured as needed for granulation, pelletization and coating processes, from laboratory to production scale with batch sizes of up to 150 kg.
A Glatt Modcos process machine is available, in which powder can be processed up to the finished tablet in a fully-automated process. A whole range of modular processing equipment is available that can be arranged into a fully-automated process line. Up to 6 processes can be performed in parallel.
What do you think is the most important unique feature?
Klaus Möller: The combination of Pharmaceutical Services’ competences in the development of formulations, processes and technology, the Innovation Center and the possibility to manufacture clinical samples under GMP conditions is, as far as we know, a range of services which only we can provide. Our technical capacities allow us to scale up from laboratory to commercial production scale seamlessly under GMP conditions. This means we accompany the customer from the initial product idea to market launch.
In which specific problems can you offer assistance?
Klaus Möller: The development of processes and formulations is a massive playing field and places the customer before a huge range of challenges. Our specialty is the development of solid dosage forms with a focus on complex formulations and we have great expertise in multiparticular systems. This is why customers frequently come to us with matters such as product and process development or even the registration of a product.






