SMED in Pharma - A Lean Approach
 By Navdeep Singh Kathuria
By Navdeep Singh Kathuria
Over a decade, changeover for equipment has garnered a close level attention as a crucial challenge in pharmaceutical industry and it further becomes a challenging task when multiple equipment has to be handled and cleaned within a defined timelines within standard specifications and defined set of procedures.
Complexity of processes and relatively longer changeover time in pharma is not an unknown reality! A businessman expects regulatory compliance with profitability and any new concept implementation within a budgeted amount to obtain a win-win situation. Balancing all these three aspects is a big challenge and for this a strong framework and a defined strategy is required within the compliance mode.
As major part of available time gets consumed in the major cleanup of the production line, only 20-30% of the remaining time is left with the operational activities which enforces the businessman to think over the necessity to implement some methodology to optimize the changeover time and increase operational efficiency keeping regulatory norms also in place and then, a word Lean comes into everyone’s mind!
Also, being a crucial part of production procedures, dereliction or careless approach in changeover can attract a major concern with respect to the quality aspects, i.e., variations in results of analysis and can raise a question on the validity of cleaning methods and inadequate procedures of the organization. Because of that only, changeovers are meticulously planned and integrated into work order planning as a critical process where each minute spent on change over represents an availability loss in Overall Equipment Effectiveness (OEE). Therefore, it becomes a necessity to optimize the change over time efficiently with all possible efforts.
If we broadly consider about the different steps of changeover process, it involves cleaning, changing the dies (the parts that shape the tablets), assembling parts, settings/ fine adjustments and qualifying the equipment/set of equipment for each product.
Before pharmaceutical industry came ahead strategically to find out the new techniques to optimize the changeover times, Toyota already had started working in 1950’s on Lean principles and came up with a structured approach on improving changeover time of their presses which they named as Quick Die Change or QDC, resulting in reducing changeover times from multiple hours to a couple of minutes in 1960s and further reduction to less than 3 minutes in 1970s. From here, the journey started, and other industrial sectors started showing their interest in acquiring lean tools and techniques to deal with.
What is SMED?
SMED, as an abbreviation, is more popular, but, in the full form, it is basically Single Minute Exchange of Dies, the process of reducing changeover or set up time of any equipment.
Father of SMED – Shigeo or Taiichi?
 Experts move in a bit of conundrum about who discovered Lean, either Shigeo or Taiichi? But if we review the background of junctures in the journey of Lean, both have equally and significantly contributed to develop this approach in their own way. Both were known for their way of implementing this approach, Shigeo was gentle, relentless in stimulating people to change for the better, while Taiichi was a bit ruthless and a hard man, who didn’t give a choice and whatever he had instructed, had to be completed anyhow by his team, with the defined results in a shortest period of time.
Experts move in a bit of conundrum about who discovered Lean, either Shigeo or Taiichi? But if we review the background of junctures in the journey of Lean, both have equally and significantly contributed to develop this approach in their own way. Both were known for their way of implementing this approach, Shigeo was gentle, relentless in stimulating people to change for the better, while Taiichi was a bit ruthless and a hard man, who didn’t give a choice and whatever he had instructed, had to be completed anyhow by his team, with the defined results in a shortest period of time.
Lean vs GMP- Complexity and solution
GMP and Lean, both are anticipated as conflicting principles to each other but understanding or representing both as equal partners can define the importance of both the concepts independently. GMP, as a statutory requirement, is focussed on the systematic approach towards quality aspects ensuring the medication delivery to the customer with highest standards while prime functionality of Lean is quality along with reducing cost by means of elimination of waste.
Another aspect is that the GMP environment of a health industry is burdened with a flow of standard validated procedures and documentation approval from regulatory authorities, which consumes unexpectedly higher timelines and any deviation from the qualified timeslots leads to the non-compliance, filing deviation and investigation compared to the Lean, where any individual can start up with generation of a single idea, the feasibility of which can be evaluated together with cross functional team and can be implemented immediately.
Lean organisation makes an effort to regularly identify and eliminate waste and recognize employee contributions by continuous process improvement, enabling increased flexibility and competitiveness. To overcome such overlapping, Regulatory Affairs, QA and Production need to work parallelly in a balancing way, on the identified improvement projects to support Lean approach without deviating from quality parameters.
About GMP
After the Sulphanilamide tragedy in US in 1937, killing more than 100 patients due to the excipient, Ethylene glycol, US Congress passed the first Drug and Cosmetic act in 1938 which led to the creation of FDA.
After a considerable period, the first text on GMP was prepared in 1967 by a group of consultants of the World Health Organisation (WHO) and was accepted by the Twenty-first World Health Assembly under the title Draft requirements for Good Manufacturing Practices in the manufacture and quality control of medicines and pharmaceutical specialties.
Considerable developments in GMP took place in next decades, and important national and international documents, including new revisions, appeared to comply with the global standards with the prime agenda of safety of the patient.
GMP has been representing a complex system of requirements and institutionalized tradition of pharmaceutical manufacturing for decades. Therefore, it can be considered as a key reason that compared to other manufacturing sectors, the pharmaceutical industry accepted Lean principles very slowly as Lean concepts were seen only as a prime motive of cost saving one.
Due to the apparent success of Lean in general manufacturing sector, a growing number of players of the pharmaceutical industry have also decided to implement Lean, so that they can accomplish their strategic goals such as decrease wait time to release a product to the market, reduce production waste, improve communication with patients and increase quality level in production and testing laboratories. Therefore, for pharmaceutical and medical device manufacturers, Lean manufacturing represents the way for significant improvement of quality and operational efficiency.
The challenge for them in turning to Lean is that all new operational procedures must be compliant with the requirements of GMP and support continuous improvement. (While Lean primarily focuses on continuous improvement and value creation, GMP focuses on ensuring safety, reliability and quality of the drug product).
Therefore, in pharmaceutical manufacturing, the methods of Lean management must be harmonised with the standards of GMP by adopting the below key themes of SMED:
1. Measure current change over time
2. Group internal and external elements
3. Convert internal elements into the external
4. Rationalize internal and external set up
First, let us understand the internal and external elements: Elements that must be completed while machine is stopped is internal, and the element which can be completed while equipment is running is called external one.
The implementation process of SMED is divided into seven stages as follows:
1. Identify the areas or processes required to minimize changeover
2. Analyse the changeover time
3. Analyse all actions performed during the format change
4. Divide the changeover time into internal and external actions
5 Optimize the internal phase of tool change (use parallelization)
6. Optimize the external phase of tool change (use parallelization)
7. Review the procedure and build up Action plans
8. Standardize the procedure, finalize with quick implementation
Following challenges are generally noted while adopting SMED tools on floor:
• Long changeover times
• Unnecessary / long / repeated movements are made by the operator and material handler
• Lack of space in the shop floor
• Disorganisation of equipment (during the changeover)
• Versatility in cleaning methods among the areas (used chemicals, tools)
• Versatility in contact times of using chemicals
For solving these problems effectively, SMED can be monitored effectively with the tools of activity cards (with parallelization), new standards, tool kits (with photo illustration), Kanban card (Weighing and measuring area), 5S (different places).
Activity card
This standardised form provides participants with details on changeover procedure. All the activities according to SMED future state are enumerated with timeframes for each participants , e.g., operator 1, operator 2 and helper.
Tool kit
A kit composed upon the exact definition of the tools needed for a changeover. Check list, photo may help in the process of standardizing. Tool kits may also be put for supporting construction work and cleaning, as maintenance tool kit and cleaning tool kit.
Kanban card
 The term comes from Japanese “sign” or “signboard”. Kanban is a signalling device that gives authorization and instructions for the production or withdrawal of items in a pull system.
The term comes from Japanese “sign” or “signboard”. Kanban is a signalling device that gives authorization and instructions for the production or withdrawal of items in a pull system.
Kanban card is a visual signal, which can possible be bright and colourful with short instructions on it.
5S
As an essential part of Lean approach, 5S helps in many aspects to maintain area and documents during audits. They are five related terms, beginning with an S sound, describing workplace practices conducive to visual control and Lean production. The five terms in Japanese are:
a. Seiri: Separate needed and unneeded items;
b. Seiton: Organisation of needed items (place for everything and everything in its place);
c. Seiso: Clean and wash;
d. Seiketsu: Cleanliness, standardization, and training for employees.;
e. Shitsuke: Following the standards, and continuous improvement.
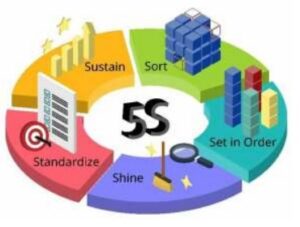 The 5S are often translated into English as Sort, Straighten, Shine, Standardize, and Sustain. One possible sixth S can be for Safety to establish and practice safety procedures in the shop floor and office.
The 5S are often translated into English as Sort, Straighten, Shine, Standardize, and Sustain. One possible sixth S can be for Safety to establish and practice safety procedures in the shop floor and office.
Gantt chart
It is a structural or graphical presentation of various activities to be executed for a project. Gantt chart is basically a task scheduling and monitoring tool for clarity and easy execution of activities planned from start to end.
Gemba walk
Gemba is a Japanese word, means, a place, where value is created. In order to truly understand and improve a process, one must go to the shop floor, observe operations, and engage with the people doing the work.
 A reasonable time for a Gemba walk should not be more than 45 minutes depending on the size of the department. A checklist can be documented afterwards to assess the observations and further acting on the same.
A reasonable time for a Gemba walk should not be more than 45 minutes depending on the size of the department. A checklist can be documented afterwards to assess the observations and further acting on the same.
5 Golden rules of Gemba
- When any abnormality arises, plan Gemba first.
- Have a check on Gembutsu (Machines, Tools, Rejects, and Customer Complaints).
- Decide temporary measures at Gemba place
- Do brainstorming to find root cause
- Standardize for prevention of recurrence.
If properly followed in a regular manner, Gemba can significantly assist in eliminating Muda (waste), Mura (unevenness) and Muri (overburden) from even complex processes and situation also.
Conclusion
Concluding this, the overlapping of GMP aspects and Lean approach exist in every pharmaceutical formulation unit at more or less frequency, which results in difficulty in acceptance of Lean methods, because of lack of a complete understanding and unawareness. Senior hierarchy in Production and Quality Assurance needs to work together to spread the understanding of Lean without deviating from the Quality standards. Wherever, any Lean concept is understood as a hindrance to the implementation of Quality matrix on floor, a joint discussion can help to sort out such issues instead of rejecting a new idea on the name of quality hindrance.
About the Author
Navdeep Singh Kathuria is General Manager – Packing, Aurobindo Pharma Ltd. He is working on implementing lean methodology along with automation of process equipment. Started his career with Tablet manufacturing, his additional area of expertise is Track & Trace systems and simplification of documentation on shop floor by means of replacing manual recording into the electronic form (EBMR).
Parle Exclusive
Articles
A Lean Approach, by Navdeep Singh Kathuria
To mitigate Regulatory Challenges in Aseptic Manufacturing, by Dr. Subrata Chakraborty
Thoughts on Sustainability Drives by Prabir K Das
















