Mastering Coating Uniformity
The Key to High-Quality Pharmaceutical Tablets
 Tablets are the most important oral dosage form in the pharmaceutical industry. Pharmaceutical film coating, which involves spraying a thin film of one or more polymers (and usually other functional excipients such as colour pigments or surfactants) that can perform a variety of functions onto the tablet, is now an important process step in the pharmaceutical industry.
Tablets are the most important oral dosage form in the pharmaceutical industry. Pharmaceutical film coating, which involves spraying a thin film of one or more polymers (and usually other functional excipients such as colour pigments or surfactants) that can perform a variety of functions onto the tablet, is now an important process step in the pharmaceutical industry.
Originally derived from dragée-pan processing, coating technology has evolved continuously over the past decades. Most coating processes are used to modify drug release, to improve drug stability against light or moisture and to mask taste. In addition, patient compliance issues play an important role, such as improved swallowability or easier identification through a different colour.
Achieving Uniformity in Tablet Coating: A Critical Factor for Quality and Performance
The quality requirements for coated tablets can also be varied, depending on the purpose of the coating. The simplest coatings require only a small minimum thickness. These are, for example, swallowing aids or simple taste and odour masking, and the coating is often colourless. Coating uniformity within a batch is of secondary importance.
Cosmetic coating, where the tablet is coloured with a pigment or dye, is the next level of complexity. The main arguments for cosmetic coating are compliance, prevention of medication errors and marketing. The quality requirements are that the tablets should be uniform within a batch and between batches, with low surface roughness and sometimes high gloss (depending on the market).
In the case of delayed-release tablets, the thickness of the coating must be thicker than that of swallowable tablets. Furthermore, the tablets must be coated with a high degree of uniformity, as disintegration and release tests in various media must be passed within narrow limits according to pharmacopeial requirements.
Coated tablets used to control the sustained release of active ingredients and tablets coated with medicinal substances are the most demanding in terms of coating quality. Very high uniformities must be achieved, otherwise the complex release profiles cannot be reproduced.
As can be seen from the introduction, the coating process is a very complex one, in which the individual process steps must be precisely coordinated.
API Coating
API coating has become increasingly important in recent years because it allows fixed-dose combinations or the combination of incompatible drugs. In addition, different drug release characteristics can be achieved by applying, for example, sustained release coatings in addition to immediate release coatings. These formulations can consist of up to four coating layers, resulting in a long processing time. To successfully develop and produce such formulations, coating uniformity is a prerequisite and a quality attribute, as coated tablets must pass the dosage unit uniformity test according to the pharmacopoeias.
Tablet Coating Process
An optimal tablet coating process – consisting of simultaneous spraying, mixing and drying processes – that meets the quality requirements of pharmaceutical production can only be achieved with the optimum selection of the correct parameters.
simultaneous spraying, mixing and drying processes – that meets the quality requirements of pharmaceutical production can only be achieved with the optimum selection of the correct parameters.
L.B. Bohle (Ennigerloh, Germany), a global technology company in the planning and implementation of machines and processes for the production of pharmaceutical solids, offers tablet coaters in laboratory and production scale.
For all its tablet coaters, L.B. Bohle relies on three design principles to achieve optimum coating results: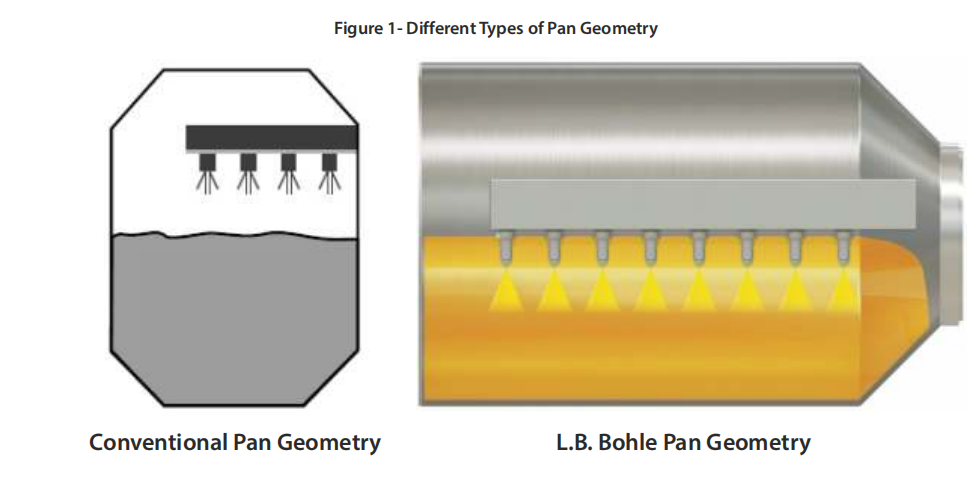
- A pan geometry with an L/D ratio of > 1 provides a large tablet bed surface area, allowing the integration of a large number of spray guns (Figure 1). Compared to other pan geometries on the market, process time can be reduced by up to 40% due to the higher throughput of coating suspension. In addition, the thinner tablet bed induces minimal shear on the tablets, allowing even very weak tablets to be coated.
2. The helical baffles inside the coating drum consist of two layers of baffles. They are responsible for continuous and homogeneous axial mixing within the tablet bed. In addition, the rotation of the drum maintains radial mixing. Both movements ensure that the tablet bed is free of dead zones.
As a result, homogeneous mixing of the tablet bed is usually achieved within a few minutes. Due to the constant tablet movement, the tablets do not experience any acceleration peaks that could cause tablet damage or even twinning.
3. The air principle in all Bohle coaters utilizes the drying capacity where it is most needed: in the tablet bed. Thus, most effectively the drying capacity of the inlet air is used without heating the rest of the coater inner parts. The inlet air is coming from below the tablet bed and is directly sucked through the rotating tablet bed into the exhaust air funnel. This setup also offers another advantage: The spray guns are not being heated during coating and remain cool. Therefore, spray losses are reduced to a minimum which leads to coating efficiencies of >97% which is particularly beneficial for API (Active Pharmaceutical Ingredient) coating processes.
Semi-Continuous Manufacturing of Coated Tablets
Continuous manufacturing remains one of the hottest topics in the pharmaceutical industry: In recent years, continuous manufacturing has become increasingly important to both pharmaceutical companies and regulatory authorities worldwide. In line with the pharmaceutical industry’s drive to make many production processes continuous, machine manufacturers are developing coaters for continuous operation. These are linked to a continuous production line and are designed to achieve the highest possible throughput.
 L.B. Bohle is one of the pioneers of continuous production, offering conventional batch coaters and the KOCO semi-continuous coater for efficient film coating processes. Together with the ROB , a fully automatic tablet container post hoist, these two machines provide an integrated solution for an efficient and fully automated coating process in pharmaceutical production environments. With its automation concept, the combination of KOCO and ROB is designed for connection to a tablet press, either for existing batch-oriented production environments or as an extension to a continuous manufacturing line.
L.B. Bohle is one of the pioneers of continuous production, offering conventional batch coaters and the KOCO semi-continuous coater for efficient film coating processes. Together with the ROB , a fully automatic tablet container post hoist, these two machines provide an integrated solution for an efficient and fully automated coating process in pharmaceutical production environments. With its automation concept, the combination of KOCO and ROB is designed for connection to a tablet press, either for existing batch-oriented production environments or as an extension to a continuous manufacturing line.
KOCO & ROB: A Semi-Continuous Coating Solution for High-Efficiency Tablet Production
The KOCO and ROB machine combination provides a turnkey solution for the smooth integration of a semi-continuous coating process downstream of a tableting process. The main application is for tableting processes that run for several hours at constant throughput. Examples are high volume batch products or continuous production lines.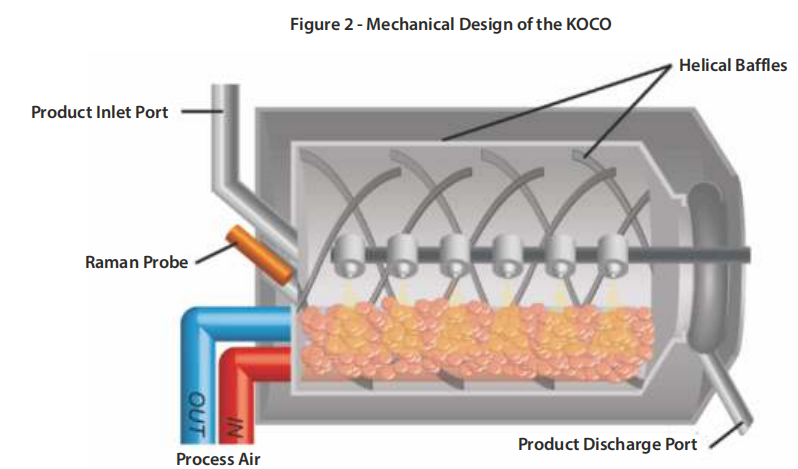
The KOCO is designed as a semi-continuous coater, ensuring reliable throughput and constant dwell time for all cores.
The process machine, as shown in Figure 2, is based on the proven Bohle design for drum coaters.
The spirals ensure uniform distribution and mixing of the tablets throughout the spray zone. In addition, the process air enters and exits the process chamber through the tablet bed, which minimises spray drying compared to a typical diagonal air flow.
The spray arm features automatic spray angle and distance adjustment and carries a total of six dual-substance spray nozzles (for KOCO 25). In addition, the spray system has recirculation and a single supply of spray nozzles to ensure uniformity and reliability in the application of the coating suspension.
The KOCO, unlike conventional batch coaters, uses a product inlet port at the top of the machine to feed tablet cores into an opening at the rear of the coating pan. Normally this port is closed and the product valve is only opened to fill the drum for a new coating cycle. The coated tablets are discharged through an opening at the front of the coater.
The process control is designed to operate in a cyclic manner, to repetitively execute the defined recipe, each execution further denoted as cycle.
Process Cycle - Phases
- Feed the coating
 pan with a given quantity of tablet cores through the inlet port.
pan with a given quantity of tablet cores through the inlet port. - Warm-up of the tablet bed, usually using the exhaust temperature as the target parameter.
- Spray the suspension with drying air active, if necessary, following a spray profile with different spray rates.
- Drying of the coated tablets.
- Cooling of the tablet bed.
- Discharge of the coated tablets through the outlet in the front door.
 Due to the design advantages of the coating pan, air duct and spray system, the KOCO can repeat the coating cycle without affecting the process and product quality. The coater is equipped with a bypass valve to keep the process air active so that the next process cycle can be started quickly after emptying and filling new tablet cores.
Due to the design advantages of the coating pan, air duct and spray system, the KOCO can repeat the coating cycle without affecting the process and product quality. The coater is equipped with a bypass valve to keep the process air active so that the next process cycle can be started quickly after emptying and filling new tablet cores.
The choice of a semi-continuous process was made to meet the requirements and expectations of the pharmaceutical industry: A truly continuous coating process is defined by tablets being fed into the process at a constant flow rate, passing through the actual process, and coated tablets continuously leaving the process at the other end. However, since the behavior of the tablet cores as they pass through the process cannot be sufficiently controlled, this results in wide residence time distributions and consequently varying exposure times of the tablet cores to the spray process.
 This leads to insufficient coating uniformity, especially for functional coatings. As a semi-continuous coater, the KOCO offers equal dwell times for all tablet cores in combination with the advantages of the helical mixing spirals.
This leads to insufficient coating uniformity, especially for functional coatings. As a semi-continuous coater, the KOCO offers equal dwell times for all tablet cores in combination with the advantages of the helical mixing spirals.
Finally, the integration of a Raman spectroscopy probe head at the rear of the coating pan can be used for in-line process monitoring. This makes the KOCO ready for PAT and improved process control using Raman spectroscopy to determine the end point of the process.
This article is contributed by L.B. Bohle (Ennigerloh, Germany).

