From Protection to Patient Empowerment
How Pharma Packaging Has Evolved
By Prabir K Das
The evolution of pharmaceutical packaging is a story of science meeting society – shaped by history, technology, regulation, and consumer needs. From the era of glass bottles and metal tins to today’s intelligent, interactive, and eco-conscious designs, packaging has transformed far beyond its original role of containment and protection. This journey reflects changing patient expectations, advances in automation, AI, and nanotechnology, and the growing emphasis on safety, compliance, and sustainability. Yet, even as global regulations tighten and digital traceability becomes the norm, challenges remain – especially for MSMEs grappling with costs, technical expertise, and regulatory complexities. As the industry looks ahead to a future of IoT-enabled and personalized medicine packaging, the balance between innovation, affordability, and environmental responsibility will define the next chapter in pharma packaging’s evolution.
Historical Evolution
Earlier most of the pharmaceutical products were using traditional packaging materials / containers, focusing more on protection of the products from external environment. Mostly glass and metal-based packaging were being used as primary packaging for best possible protection. Oral solids were being packed mainly in bulk counts and were being dispensed from the containers as per the doctor’s prescriptions. Liquid products were being packed in glass bottles. Often compounders at doctor’s clinic were preparing liquid oral formulations by mixing chemical ingredients as per doctor’s recipe. Concept of unit dose packaging for oral solids was slowly catching up and strip packaging was very common and convenient during that time. Injectables were mostly in glass containers. Viscous formulations were mostly in metal containers (tubes).
 Prabir K Das is an alumnus of the first batch PG students from Indian Institute of Packaging (1985-1987), Mumbai. He has served the industry for over 36 years, majorly in pharmaceutical industry, including Ranbaxy Laboratories and Mylan Laboratories. He has exposure in packaging design and development, technology transfer, artwork development and processing, change management, automation, standardization and harmonization, COGS (cost and quality) improvement exercises, track and trace (serialization) project, documentation, and other activities related to packaging and labelling needs as per global standards.
Prabir K Das is an alumnus of the first batch PG students from Indian Institute of Packaging (1985-1987), Mumbai. He has served the industry for over 36 years, majorly in pharmaceutical industry, including Ranbaxy Laboratories and Mylan Laboratories. He has exposure in packaging design and development, technology transfer, artwork development and processing, change management, automation, standardization and harmonization, COGS (cost and quality) improvement exercises, track and trace (serialization) project, documentation, and other activities related to packaging and labelling needs as per global standards.
He is always an active participant and attended many national and international seminars and conferences either as an independent presenter or as one of the panel members. He has also contributed many insightful articles related to packaging and labelling in leading magazines / journals.
There are multiple factors which influenced not only packaging forms and their design, but also many pharmaceutical formulations. Migration and mobility of people, environmental and climatic changes, relatively smaller families, change in food and drink habits, fast moving and changing lifestyle, ease of dispensing and administering, expectation for faster relief and recovery, and so on.There are many technology-driven packaging innovations which has changed the entire landscape of the industry. A few technologies are now superseded with newer versions. While a few are investment intensive, others are easier to adopt.
Then globalization and consumerization rapidly started spreading during 90s. Mobilization of people and change in their life style further triggered its fast expansion and enrichment. This was happened primarily to support mass scale distribution of various commodities across the geographies and to reach large section of population around wider section of societies for their personal and healthcare needs.
Healthcare and pharma industry was no exception as it is directly linked to health and wellbeing of people and their families. Various stages of industrial revolution helped to support evolution and enrichment of technologies for product manufacturing, packaging, testing, distribution and documentation. Massive evolvement of pharma packaging also started during this period along with other industries. The salient transformation in pharma industry (product-process-packaging) can be summarized as below:
- Evolution to Industrial Revolution – Local to Global – Large wholesale pack to small user-friendly consumer pack.
- Scaling Up – Quantity with Quality – Reproducibility & Consistency – Boosting Productivity supported with quality and stability.
- Manufacturing and Packaging – Testing and Releasing – Data and Documentation compliance.
- Support Brand Promotion and Brand Protection – Prevent Counterfeiting or Cloning, Prevent Theft and Diversion.
- Storage, Handling and Transport – Logistics and Distribution – Safety, Security and Traceability.

Traditional glass/metal containers vs. modern packaging
Glass and metal-based packaging were being used for many pharmaceutical products due to their strong barrier properties and easy availability during that period. However, with the advancement of technologies, other materials (mainly plastics / polymers and composites) also developed to provide required protection to the products. Conversion of glass and metal was high energy consuming industry in comparison to that of plastics / polymers and composites.
Glass / metal-based packaging was heavier in weight in comparison to plastics / composite based packaging. Process waste, transit damages and distribution cost were higher in case of glass and metal-based packaging. All these factors made plastics / composite-based packaging a cheaper alternative to glass / metal-based packaging. However, still glass and metal-based packaging are being used for certain products in the industry where suitable alternatives were either not yet studied or stability / quality is not encouraging with the alternate packaging.
Consumer-Centric Shift
How lifestyle changes and patient expectations shaped packaging
There are multiple factors which influenced not only packaging forms and their design, but also many pharmaceutical formulations. Migration and mobility of people, environmental and climatic changes, relatively smaller families, change in food and drink habits, fast moving and changing lifestyle, ease of dispensing and administering, expectation for faster relief and recovery, and so on. Formulations and packaging, both have been customized as per the need from people of various strata of the society.

Impact of compliance packaging
With the development of advanced medication / formulation and their dosage regime, it was found that certain medicines require to be taken as per the doctors’ prescription for a fixed time period for better and faster recovery. Calendar packs were developed to ensure that the patients take certain medicine without any interruption / error. There are few variants like combination products with morning-evening administration, weekly packs with defined day (Monday, Tuesday, etc.) and monthly packs (week 1, week 2, etc.). Such packs ensure compliance by adhering to the desired dosage regime. Regulatory agencies monitor such functionality while approving such medication for manufacturing / marketing for the patients.
 Similarly, there are medicines which require protection from misuse by the children. Packs for such medicines are designed in such a way that children cannot easily open the container-closure system to access the medicines. These packs are known as child-resistant (CR) packs, which are certified by authorized testing houses. They organize the CR testing as per regulatory / quality approved protocols to certify the CR functionality of the specially designed packs (container-closure systems).This is also monitored by the regulatory agencies while reviewing and approving the medicine for manufacturing / marketing for the patients.
Similarly, there are medicines which require protection from misuse by the children. Packs for such medicines are designed in such a way that children cannot easily open the container-closure system to access the medicines. These packs are known as child-resistant (CR) packs, which are certified by authorized testing houses. They organize the CR testing as per regulatory / quality approved protocols to certify the CR functionality of the specially designed packs (container-closure systems).This is also monitored by the regulatory agencies while reviewing and approving the medicine for manufacturing / marketing for the patients.
Technological Advancements
With rapid globalization, consumerization and industrialization, all types of industry sectors started reforming to deliver volume and variety for increased quantity with consistent quality. Global competition set new challenges to cope up with existing infrastructure. Business houses realized need of upgrading various processes to enhance quantity with quality to catch up competition and to sustain their very existence.
Even though there were many internal challenges, process automation became inevitable and industry started adopting newer technologies to boost productivity and quality standards. Slowly mechanical and electrical systems were being upgraded with electronics and nano-technology. Analog systems were gradually converted to digital systems. Hardware-software combination elevated the efficiency of wide range of equipment and machineries. Process control and quality control also simultaneously upgraded as a complementary necessity.
electronics and nano-technology. Analog systems were gradually converted to digital systems. Hardware-software combination elevated the efficiency of wide range of equipment and machineries. Process control and quality control also simultaneously upgraded as a complementary necessity.
Further enhancement was facilitated through robotic activities to minimize human intervention in managing many critical and intricate processes. Human-induced variability was eliminated through automation and robotics.
All these transformations were simultaneously brought and applied in pharma packaging too. This helped not only to enhance volume, but also to upgrade quality to match global standards. The transformation process further evolved with newer processes like Augmented Reality (AR), Virtual Reality (VR), Machine Learning (ML) and Artificial Intelligence (AI) through various software based digital and wireless technologies.
All these processes are gradually deployed not only to enhance process efficiency and but also to improve quality standards as per contemporary global guidelines to compete and to comply with regulatory and quality norms.
 Various packaging and labelling designs are now being finalized using latest digital technologies. Right from product-packaging compatibility and simulation of stability studies are based on large data on materials and container-closure systems. Primary packaging is being decided by knowing the product sensitivity and basic packaging material properties.
Various packaging and labelling designs are now being finalized using latest digital technologies. Right from product-packaging compatibility and simulation of stability studies are based on large data on materials and container-closure systems. Primary packaging is being decided by knowing the product sensitivity and basic packaging material properties.
 Container-closure systems are being finalized considering optimum head space, minimum permeation, low migration and leaching. Label size and die-line are derived based on the container-closure data.
Container-closure systems are being finalized considering optimum head space, minimum permeation, low migration and leaching. Label size and die-line are derived based on the container-closure data.
Graphic designs on labelling are also being digitally derived through software along with various overt and covert features, which are being digitally generated and are being incorporated in labelling for brand promotion and brand protection. Inclusion of product-pack specific linear barcode and 2D data matrix code are also being generated through digital designing process.
data matrix code are also being generated through digital designing process.
Many product labels are now available with such features which facilitate identification and authentication through digital scanning. Interactive features are also available on labels to provide information related to the product and its license holder / manufacturer, through a simple scan.
Sustainability & Eco-Friendliness
Use of biodegradable/compostable materials
Over the years packaging design and development has been happening unidirectionally, focusing mainly on product stability and its quality. This has brought many alternative packaging replacing the conventional glass and metal-based packaging.
Due to increased volume / variety of various dosage forms, introduction of smaller disposable packs, evolvement of lifestyle deceases, increased access and dependency on medication by the people, and similar causes compelled us to large scale use of medications, disposing its packaging. This has generated another major challenge against protection of environment. Such alternative packaging materials are neither easily recycled nor easily degrade. The situation further aggravated due to lack of awareness by the users.
Now time has come to also focus on a healthy sustainable environment by judicious use of alternate packaging materials, which are easy to recycle and /or degrade faster, without compromising product stability and its quality. It is not easy task to find biodegradable / compostable materials which can also ensure stability and quality of sensitive medications. This is a long journey, involving alternate material and container-closure development, generation of fresh long term / real time stability data, regulatory filing / review / approval, etc. Nevertheless, the process has started in this direction and development is happening in stages due to fast changing technological advancements. Simultaneously, awareness building exercise is also growing fast, enabling people to respond positively to this concern. Regulatory agencies also started controlling it for use of certain percentage of alternate material to address the concern.


Circular economy and recycling challenges
Circular economy is a concept of 5R – Reduce, Recycle, Reuse, Refuse and Reform. In recent years packaging waste has become a hot topic for attention and discussion. However, it is comparatively less with pharma packaging. Nevertheless, to safeguard the environment and to minimize the negative impact imposed by packaging waste, pharma industry also started focusing optimization of packaging materials and use of recyclable materials which will support the theory of circular economy and contribute to reduce the burden to the environment.
Recycling of single component packaging materials is much easier in comparison to composites (combination of two or more materials). Considering the volume and variety of population spread across a wide range of localities, even collection of single component packaging materials for recycling is still a major concern.
 Barring single component glass, metal and plastic containers, many pharma packaging often uses different types of composites for better protection of the products through hermetic sealing of the container-closure systems. Such composite packaging also supports unit dose consumer friendly options with dispensing ease of the products. Separation of individual components from such composite materials is technically and economically not feasible and hence recycling and reformation of such materials are practically impossible.
Barring single component glass, metal and plastic containers, many pharma packaging often uses different types of composites for better protection of the products through hermetic sealing of the container-closure systems. Such composite packaging also supports unit dose consumer friendly options with dispensing ease of the products. Separation of individual components from such composite materials is technically and economically not feasible and hence recycling and reformation of such materials are practically impossible.
Other options like reuse and refuse with pharma packaging are also limited, especially the primary packaging. Major possibility is with secondary and tertiary level of packaging, where down gauging, minimizing or eliminating certain elements can be done through adequate studies and trials to support refuse and reduce options.
Balancing sustainability with regulatory compliance
Considering the environmental burden due to packaging waste, various regulatory agencies drafted and circulated a few norms to control the packaging waste so that our mother earth can sustain an ecologically stable condition for all living beings to survive in a healthy environment.
Business houses also started planning and budgeting to optimize material usage, to simplify operations and to control process wastage. Such initiatives not only focus on packaging, but also on other areas of entire business operations. Regulators started reviewing the initiatives taken on various sustainability projects and how they are contributing to mitigate the concern.


Regulatory Environment
Key global regulations (USFDA, EMA, CDSCO, WHO) and their impact
Even though there are different regulatory agencies with different guidelines based on the demographic requirements, all quality and regulatory norms are primarily derived based on safety and security of the patients, irrespective of the country and region.
Consistency, reproducibility, continuity, productivity and flexibility are the prime focus areas in the entire business process. All operating systems and procedures along with their documentation are designed based on these focus areas. Regulatory agencies review and certify these based on the authenticity and robustness of the processes and how they are performed and documented with honesty and integrity. Any kind of nonconformity is not allowed or accepted by the regulatory agencies which may directly and/or indirectly impact the patient safety and security.
Serialization compliance across different geographies
With the rapid growth of the industry, a parallel route also evolved to grab a large share of the highly lucrative profit margin and imposed critical threat, especially to the pharmaceutical and healthcare sector, staking human health / lives. To mitigate the risk various security features like tamper evidence, anti-counterfeiting, traceability and authentication were introduced for product protection and supply chain security.
Serialization is one such feature which supports tracking and tracing of the product-pack across the entire distribution cycle. It also helps as an anti-counterfeit and authentication feature. Like regulatory guidelines, serialization requirement also varies based on the country / region. However, use of a 2D data matrix code on individual pack is common and its identity differs from pack to pack for the same batch, making it unique. The code is scanned for verification before release / dispensing of the product-pack.
The rulings are already effective in a few countries / regions and all exporters are complying to the norms for exporting the goods to these countries / regions. Even though it is an expensive feature, packaging lines are automated and equipped to do this on-line, during product packaging operations, thereby complying with the regulations of specific country / region.
Supply Chain & Track & Trace
Real-world implementation challenges
 Being a relatively new concept involving new technology deployment, there were many implementation challenges faced by the industry. It required automated linear operations for on-line execution and control, and a secured supply chain network which can ensure delivery of the right product in right quantity at right place on right time with right quality.
Being a relatively new concept involving new technology deployment, there were many implementation challenges faced by the industry. It required automated linear operations for on-line execution and control, and a secured supply chain network which can ensure delivery of the right product in right quantity at right place on right time with right quality.
 Relatively old business houses had limitation of space, budgetary constraint, non-availability of knowledgeable and skilled manpower, impact on product costing, logistics and distribution constraint, etc. Nevertheless, service providers helped the industry to adopt the process as per specific country requirements and provided desired solution to comply seamless supplies with the overseas countries. Majority of the exporters upgraded operations to comply the norms.
Relatively old business houses had limitation of space, budgetary constraint, non-availability of knowledgeable and skilled manpower, impact on product costing, logistics and distribution constraint, etc. Nevertheless, service providers helped the industry to adopt the process as per specific country requirements and provided desired solution to comply seamless supplies with the overseas countries. Majority of the exporters upgraded operations to comply the norms.


Cost vs. benefit of serialization for small manufacturers
Serialization is a costly operation due to facility upgradation with linear automated packaging lines, and it contributes in the unit cost. However, it is a compliance driven requirement which helps end-to-end traceability with identification and authentication of the products, and safety and security to the patients.
Manufacturers from MSME sector affected the most due to inadequate fund, limitation of space and trained workforce. Over the years the situation is improved a lot, but still a long way to become normal as was in pre-serialization phase.
 Cost of quality and cost of compliance took precedence over cost of product. But it is now must for survival in the global competition and sustain the very existence in the marketplace. Nevertheless, looking into long term benefit of serialization to prevent cloning / counterfeiting, pilferage / theft and supply disruption / diversion, they are adopting it in phases as needed by the importing countries.
Cost of quality and cost of compliance took precedence over cost of product. But it is now must for survival in the global competition and sustain the very existence in the marketplace. Nevertheless, looking into long term benefit of serialization to prevent cloning / counterfeiting, pilferage / theft and supply disruption / diversion, they are adopting it in phases as needed by the importing countries.
Challenges for MSMEs
As already explained above, manufacturers from MSME sector are adversely affected due to inadequate fund, limitation of space, limited access to technical know-how and trained workforce to absorb the new technology and manage the operations. They always try to minimize the overhead cost to sustain their business flow and this new requirement always leads to an increase in overhead cost, thereby reduces the profit margin. So, adapting new  packaging requirements is burdensome and often leads to reluctance. When it becomes mandatory, compromise happens on final quality of the product, leading to noncompliance.
packaging requirements is burdensome and often leads to reluctance. When it becomes mandatory, compromise happens on final quality of the product, leading to noncompliance.
Government can support the industry by providing financial, technical assistance and can help in building a pool of skilled manpower. Possibilities like reduction of equipment cost through indigenization, reduction of tax burden on such equipment, provision for interest free loan or loan with nominal interest, are few options.
Similarly, deputing technical experts for efficiently managing the serialization project with low investment, select and train people who can manage the project independently, guide and train the work force to upgrade their skill set so that industry can utilize these resources to comply with the requirements.
Cost-Benefit Analysis
Costly innovations proved beneficial
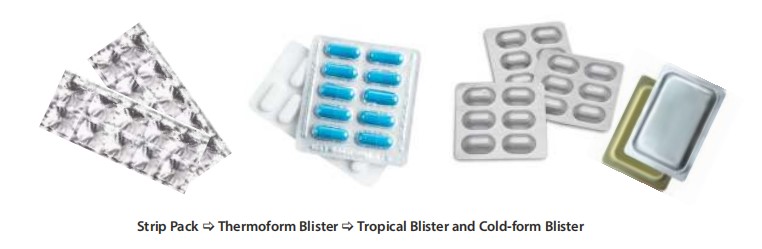
Consider the unit dose blister pack with cold form materials for solid dose formulations. The investment on equipment is higher and comparatively it is costlier than conventional PVC-based thermoform blister. However, most of the sensitive drug formulations now use cold form blister pack, considering longer stability of the product in this pack variant. Even though the pack size is relatively larger, appearance of cold form pack is better.
Similarly, consider unit dose prefilled syringe pack for injectables. Considering its convenience and ease of administration, it is well accepted by the users even though it is costlier than conventional glass ampoule packs. There are many such technology driven packaging innovations which has changed the entire landscape of the industry. Few technologies are now superseded with newer versions. While few are investment intensive, others are easier to adopt. Here is how the landscape has transformed over the years:
- Strip Pack -> Thermoform Blister -> Tropical Blister and Cold – form Blister
- Standard design -> Customized design like Calendar pack / Compliance pack
- Glass Bottle -> Plastic Bottle -> Flexible Sachet and Pouch pack
- Glass Ampoule / Vial -> Blow-Fill-Seal pack and Prefilled Syringe pack
- Aluminium Tubes -> Plastic or Laminated Tubes
- Metal Closures -> Plastic Closures with induction sealing
- Normal Screw Closure -> Child Resistant closure and Closure with in-built desiccants
- Tear off or tear down closure -> Flip off or flip top seals
- Conventional Closure -> Single dose and Metered dose dispensers
- Conventional glass or metal container -> Aerosol containers
- Cut & pre-gummed label -> PS Sticker label, Shrink label, Multilayer label, in-mould labelling
- Custom designed ancillary items – Plugs / Measure cup / Dropper, Drug delivery / Dispensing / Administration devices.
Case studies on failed or obsolete technologies
Tropical blister pack was introduced with high expectations. However, considering high investment, packaging line length, cost of pack, convenience to the user, it could not sustain long time and replaced with cold form blister pack with adequate stability data. It also replaced the conventional strip packs for many products.
Similarly, introduction of plastic bottles replaced conventional glass bottles for oral solids and liquid orals, plastic containers replaced metal containers for cream / ointment packs, glass bottles for infusions replaced with plastic BFS packs / composite laminated pouches, plastic / laminated tubes replaced many aluminium tube packs, plastic closures replaced metal closures, and so on.
Future Outlook
With the fast evolvement of automation, adoption of digital technology and wireless communication, many aspects are being improved to ease our day-to-day activities and usage patterns. Pharmaceutical product-packs are no exception and adopted such features for user convenience. Personalized packaging for medicines uses customized designs, smart technology, and unique formats to improve patient adherence, safety, and engagement. Such designs and features are focused on the individual patient’s conditions and needs.
While a few packaging are technically designed for ease in its usage, many are managed through its labelling. Selection of user-friendly text / fonts, visually different colour coding and use of digital features make the labelling interactive and helps the patient for correct administration of the medicine with punctuality.
Through digital features user can access product information, usage instructions in their preferred languages and / or a video demonstration also. Use of 2D / QR codes, NFC tags and microchips in packaging and labelling enables the product-packs more interactive with real time tracking and tracing.
How packaging might look in the next 10-15 years
With the rapid growth of digital electronics, wireless technology, artificial intelligence and IoT, packaging features are being enriched to make it friendly with the next generation people.
Mobility of new generation people have increased multi-fold and they are largely dependent on many on-line activities with handheld mobile devices / laptops / desktops. These devices can manage majority of the activities through different application software installed in them.
There is a steady growth of elderly people due to upsurge in life span and they require medical care and attention. Doctors can be consulted on-line and medicines can be procured on-line instead of physically visiting to doctors and pharmacies. People can even remotely control elderly patients for their medication need through interactive CCTV camera installed in their houses.
Similarly, there is large population of children who requires a little motivation for timely medication and this is facilitated through digital graphics and virtual imaging through packaging and labelling designs. All these requirements helped developing such model of working with good outcome. We are now transitioning and over a period of time next generation people will be more digital savvy to access the full benefits.
It is not easy task to find biodegradable / compostable materials which can also ensure stability and quality of sensitive medications. This is a long journey, involving alternate material and container-closure development, generation of fresh long term / real time stability data, regulatory filing / review / approval, etc.


