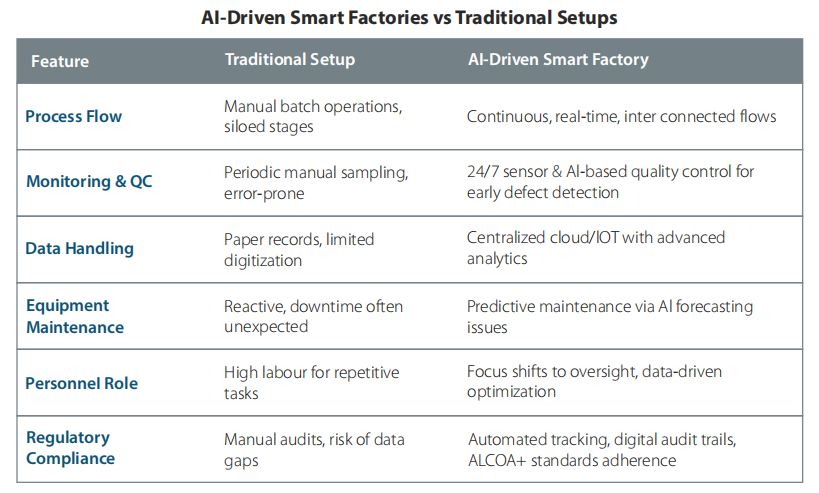
AI-Driven Smart Factories vs. Traditional Setups
The pharmaceutical industry is undergoing a transformative shift with the emergence of AI-driven smart factories, revolutionizing traditional manufacturing practices. Unlike conventional setups that rely heavily on manual intervention and fixed production workflows, smart factories leverage artificial intelligence, machine learning, and advanced analytics to enable real-time decision-making, predictive maintenance, and process optimization. These intelligent systems enhance efficiency, reduce human error, and improve compliance with regulatory standards. Traditional pharmaceutical manufacturing, while proven and reliable, often faces limitations such as longer downtimes, higher operational costs, and slower adaptability to change. In contrast, AI-powered setups facilitate greater agility, scalability, and data-driven insights, enabling manufacturers to respond quickly to market demands and maintain consistent product quality. As the industry faces increasing pressure for faster time-to-market and stringent quality standards, AI-driven smart factories represent a promising evolution toward more resilient, responsive, and cost-effective pharmaceutical production environments, redefining the future of drug manufacturing. Dr. Ranjana Pathak, who oversees Lupin’s global quality and Pharmacovigilance, speaks to Pharma Machines & Technology in an exclusive interview.

Dr. Ranjana Pathak is a seasoned pharmaceutical professional with over three decades of global experience. Her expertise lies in quality and related functions, and she currently oversees Lupin’s global quality and Pharmacovigilance.
Before joining Lupin, Ranjana held the position of Global Head for Quality and Pharmacovigilance at Dr. Reddy’s. During her tenure, she was a key member of the Dr. Reddy’s Management Council, contributing to several positive outcomes for the company.
Her leadership journey extends across various branded and generic pharmaceutical companies, including Cipla, Actavis, Endo, Zenith Goldline, and Thames Pharmacal.
Ranjana’s academic achievements include a Doctorate in Health Administration from the University of Phoenix, USA, an MBA from Dowling College, New York, a Post-Graduate Diploma in Pharmaceutical & Chemical Analysis from Sophia College, Mumbai, and a B.Sc. (Hons) in Chemistry from Mithibai College, Mumbai. Additionally, she has received specialized training in pharmaceutical and biologics leadership at Harvard University.
It’s a pharma executive’s dream, to be fully automated and digitized plus digitalized with all the bells and whistles in the shop floor and labs. In many of our parlance today it is referred to “smart factories and smart labs”.
Q. AI-driven smart factories require substantial investment. Do you think the cost justifies the potential benefits?
This is a pharma executive’s dream, to be fully automated and digitized plus digitalized with all the bells and whistles in the shop floor and labs. In many of our parlance today it is referred to “smart factories and smart labs”. We know most of the problems arise because someone did not do something in time, etc, etc… We have a fancy heading/banner for these errors and all of this is couched under Human Error. If investigated further we find that these errors did not occur due to the person (operator or analyst), it was either inherent in the process or the test method. The process may not have delineated all steps clearly, or may not have been practical, ergonomically difficult to execute, or simply not written in sufficient detail… this list is long. With a Smart Factory, all processes would be automated, chances of error become minimal. It becomes a button or click away.
Q. What are the biggest financial barriers to transitioning from a traditional setup to a fully automated smart factory?
As I said above, it’s a practitioners dream – but a very expensive dream. The investments are substantial; they will range in millions of USD, depending on the factory. It is not for every factory or lab.
The ROI has to be calculated in sufficient detail to understand the feasibility vs. the benefit. Cost/benefit ratio has to be calculated.
 It’s a practitioners dream – but a very expensive dream. The investments are substantial; they will range in millions of USD, depending on the factory. It is not for every factory or lab.
It’s a practitioners dream – but a very expensive dream. The investments are substantial; they will range in millions of USD, depending on the factory. It is not for every factory or lab.
Q. What advice would you give to pharmaceutical companies weighing the decision between traditional and smart factory setups?
My recommendation would be to assess the opportunities such as:
- Do I need to increase throughput?
- Do I have a workforce that is constantly changing (high attrition) or unskilled? It’s important to be mindful of all the nuances associated with manpower. If your firm needs to protect their intellectual property, how do you get around that?
- Are your current processes very long and cumbersome?
Q. Given India's cost-sensitive market, does full automation make economic sense, or does a hybrid model work better?
This would depend on each individual operation or factory. Also, we cannot have a narrow view when these calculations or discussions are happening. There are times when you cannot put a dollar value on the savings. For example, you may be able to retain your people because you have better processes that are automated, etc., and there is “delight” in working.
There are times when you cannot put a dollar value on the savings. For example, you may be able to retain your people because you have better processes that are automated, etc., and there is “delight” in working.
Knowledge is power. It comes down to “show me and I will follow.”. The distrust comes from a lack of knowledge and a lack of patience to try something new, cutting-edge, etc. People are uncomfortable with the “new.
Q. AI-driven smart factories require highly specialized skill sets. Do you think the Indian pharmaceutical workforce is ready for this shift?
 India is gifted with a very intelligent workforce, though it may not be adequately skilled. Here, industry and academia have to work together to produce a skilled workforce. This is not easy, but nothing important in life is easy.
India is gifted with a very intelligent workforce, though it may not be adequately skilled. Here, industry and academia have to work together to produce a skilled workforce. This is not easy, but nothing important in life is easy.
Q. How do we address resistance from experienced quality control professionals who may distrust AI-based decision-making?
Knowledge is power. It comes down to “show me and I will follow.” The distrust comes from a lack of knowledge and a lack of patience to try something new, cutting-edge, etc. People are uncomfortable with the “new.”
Q. How do you see workforce roles evolving in AI-driven pharmaceutical manufacturing?
I think if organizations are serious about what they do and want to boost productivity, reduce errors, and remain profitable, they have no choice but to find new solutions to tackle yesterday’s problems by adapting/embracing the possibilities offered by AI platforms.
I think if organizations are serious about what they do and want to boost productivity, reduce errors, and remain profitable, they have no choice but to find new solutions to tackle yesterday’s problems by adapting/embracing the possibilities offered by AI platforms.
Q. Do you believe the shift toward AI-driven automation will lead to job losses, particularly in the quality assurance domain?
There was fear back when the Industrial Revolution started that man would be replaced, and this fear has continued. I believe jobs will change; people’s minds will be used.
All repetitive tasks will be replaced by AI-driven automation, and frankly, I believe they should. There will be new jobs created that we cannot imagine, and different domain knowledge will be developed that perhaps many cannot envision.
Q. How do we ensure a balance between human expertise and AI-driven quality control in pharmaceutical manufacturing?
 This will be different for different companies; one may not need to balance this. It is not an either/or scenario. Jobs that don’t require judgment or are repetitive/commodity-type work must be replaced by AI-driven tools. Humans should be used to do what these tools cannot. In the area of pharmacovigilance, many companies are trying, but I am not aware of any that has cracked it 100%, demonstrating that humans are still needed.
This will be different for different companies; one may not need to balance this. It is not an either/or scenario. Jobs that don’t require judgment or are repetitive/commodity-type work must be replaced by AI-driven tools. Humans should be used to do what these tools cannot. In the area of pharmacovigilance, many companies are trying, but I am not aware of any that has cracked it 100%, demonstrating that humans are still needed.
Q. Is there a risk that over-reliance on AI in quality assurance might lead to unforeseen failures that could result in costly regulatory penalties?
I look at it the other way – let us use AI-based tools where the manufacturing is robust and no batch fails because the right material from a qualified vendor has been used. The batch manufacturing processes are well defined in algorithms to eliminate errors. All this will make QA’s job easier and more fulfilling. They would spend their time on meaningful review, assessment, and sound decision-making, instead of chasing their colleagues to sign, date, investigate, find root causes, etc.
Q. Are there hidden costs in AI implementation – such as ongoing software validation, cybersecurity, and regulatory audits – that companies might underestimate?
Yes, there will be cybersecurity issues, license fees, skilling of manpower, and many other hidden costs one must explore.
Q. AI systems depend on massive datasets – how do we ensure that data used in AI-driven quality control is accurate, unbiased, and tamper-proof?
Validation should eliminate this; however, the deeper intricacies will have to be examined by engaging SMEs.
Q. AI-driven decision-making in drug manufacturing raises ethical questions. Who should be accountable if an AI system fails to detect a critical quality issue?
This is a broad question. In manufacturing and lab testing, I don’t see any compromise on ethics. All of this has to be designed correctly, with the right team in place who has the expertise. It cannot be done otherwise.
 AI-driven automation contributes to sustainability in pharmaceutical manufacturing by optimizing resource use, reducing waste, and improving energy efficiency. AI can predict equipment maintenance needs, minimizing downtime and unnecessary replacements. It enhances process control, ensuring precise material usage and reducing batch failures – thereby cutting down on raw material waste and disposal costs. Additionally, AI helps in designing greener processes by analyzing large datasets to identify more efficient and less polluting methods. Overall, it supports a leaner, more environmentally responsible manufacturing approach without compromising quality or compliance.
AI-driven automation contributes to sustainability in pharmaceutical manufacturing by optimizing resource use, reducing waste, and improving energy efficiency. AI can predict equipment maintenance needs, minimizing downtime and unnecessary replacements. It enhances process control, ensuring precise material usage and reducing batch failures – thereby cutting down on raw material waste and disposal costs. Additionally, AI helps in designing greener processes by analyzing large datasets to identify more efficient and less polluting methods. Overall, it supports a leaner, more environmentally responsible manufacturing approach without compromising quality or compliance.
Evolving regulatory requirements significantly influence the approach to automation and AI in manufacturing by demanding greater transparency, traceability, and compliance. Manufacturers must ensure that AI-driven systems are validated, auditable, and capable of producing data that meets regulatory expectations (e.g., FDA’s 21 CFR Part 11 or EU’s Annex 11). This pushes companies to design automation with built-in quality assurance, robust data integrity, and human oversight. As regulations increasingly acknowledge the role of AI, manufacturers must stay agile – adapting their systems and processes to remain compliant while leveraging the efficiencies and precision that AI can offer. In short, compliance is no longer an afterthought; it’s built into the automation strategy from the ground up.