
Automation does not always result in success, and poor automation can be as damaging to the pharmaceutical organization as no automation at all. This article addresses what happens when automation goes wrong and how mistakes can be avoided by adopting a risk-centric approach
A simple term, ‘automate’ means to run or operate (something, such as a factory or system) by using machines, computers, etc., instead of people to do the work. Hence, the implementation of automation technologies, techniques and processes improve the efficiency, reliability, and/or speed of many tasks that were previously performed by humans. Expanding upon this concept, ‘what automation is’ in the pharmaceutical process, a simple example is with an automatic control loop (1). Here a controller compares a measured value of a process with a desired set value and processes the resulting error signal to change some input to the process, in such a way that the process stays at its set point despite disturbances. Other examples include (2):
- Laboratory automation: This can include systems for automated analysis processes.
- Pharmaceutical production: This can include assemblies and systems for modular and scalable plants. Drug production is divided into two main production steps:
a) Active ingredient production – either precision chemistry with relatively low hygiene requirements or biotechnology with high hygiene requirements.
b) Production of the actual form – normally with high hygiene requirements.
3. Filling and Packaging: There are different levels of hygienic requirements for filling and packaging depending on the drug and the form of dispensing. Nevertheless, they invariably require:
- high throughput
- corrosion resistant materials
- hygienic design
Hence, workplace automation is a means of combining a series of simple, repetitive tasks to create a straightforward, streamlined flow of operations, without the risk of human error. Utilizing the benefits of automation is one of the essential elements of industry 4.0. Drivers for automation also include economic pressures, such as business disruptions, safety concerns, regulatory requirements, customer service, financial problems, and competition. Each of these triggers at least conditions the pharmaceutical company to consider pursuing workflow automation.
Thus, for pharmaceuticals and healthcare, automation is no longer a question of ‘if’ but rather one ‘when’. This is not to say that automation does not exist in some form or another, since automation is, on one level, merely the extension of labor-saving device to replace something that was once performed by a human. A thermostat on a radiator is a case in point. What is meant by modern day or future-state automation is automatic control in the use of various control systems for operating equipment such as machinery, processes, and production systems. To achieve this insights into data are required together with the tools to interpret them and draw inferences (hence automation connects with big data analytics and artificial intelligence) (3).
Why automate?
By automation, this refers to the application of technology by which a process or procedure is performed with minimal human assistance, leading to a different form of socio-economic organization of work. In other words, automation is concerned with execution of a function by a machine (such as a computer or a robot) that was previously carried out by a human. Automation is additionally a means of reducing human error. Automation need not remain the wholescale replacement of human activity by a machine, for there can be an interaction between the two: Hence, automation may be joined with a human to control complex situations, which creates a human–automation system (4). A conceptualization of the generic dual-process framework of human reasoning and decision making is presented in Figure 1. 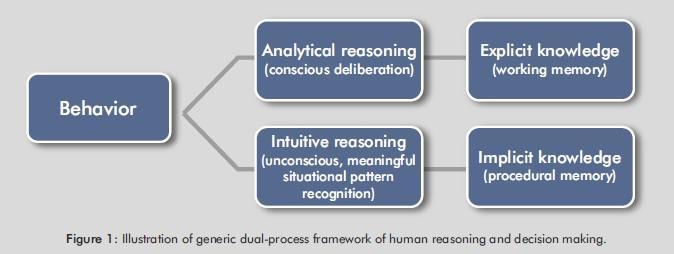
In Figure 1, analytical cognition involving conscious deliberation (also explicit knowledge and declarative memory) is shown on the left side of the diagram, and intuitive cognition involving meaningful situational pattern recognition (also implicit knowledge and procedural memory) is shown on the right side of the diagram. In addition, higher levels of automation can predict the likelihood of failures and therefore allow for preventive maintenance to be used.
Some pharmaceutical and healthcare firms are further ahead in wider-scale and far-reaching automation projects. Many of these early adopters of technology solutions are already seeing a return on investment, and a divide is forming between them and the so-called ‘digital laggers’. Even the smallest applications of automation have the potential to make a significant difference to an organization’s daily operations. As examples, many pharmaceutical companies have been integrating automation into specific processes such as drug development, serialization, anti-counterfeiting, and with processing. Manufacturing examples, automation has become prevalent in processes such as kit assembly, sortation, machine tending, and packaging (5).
The advantages of introducing automated operations include higher productivity, improved reliability, greater availability, improved performance, and reduced operating costs. When undertaken correctly, automating operations yields will lead to a return on investment in the short term. However, automation does not always result in success, and poor automation can be as damaging to the pharmaceutical organization as no automation at all. This article addresses what happens when automation goes wrong and how mistakes can be avoided by adopting a risk-centric approach.
Examples of automation
An example of automation is with Robotic process automation (RPA).The application has been the hallmark of operational improvement for several businesses, with some able to install the operative capacity of 230 full-time employees at 30 percent of the cost using the tool (6).Central to the RPA implementation argument is automation. Appropriate RPA software automates repetitive, rules-based processes usually performed by people sitting in front of computers. By process, this describes how systems and technologies are used, the interactions between people and systems, and the interactions between providers and patients. Technologies often help by standardizing and systemizing the possible actions that interacting humans can and should take (7).  This includes addressing rule-based digital tasks such as filling in the same information in multiple places, re-entering data, or copying and pasting. In achieving this, the technology does not use actual ‘robots’ in the traditional sense, but rather a concept based on metaphorical software robots or on artificial intelligence (or digital workers). In particular, RPA usage has been increased by manufacturing firms in order to minimize the risks associated with the global corona virus situation. Therefore, demand for robotic process automation is anticipated to elevate once the corona virus pandemic abates. Given RPA provides additional layers of efficiency and visibility into the data infrastructure or businesses, companies who fail to modernize are at risk of losing out on significant returns. Scalability and flexibility are often called out as the key characteristics that attract businesses.
This includes addressing rule-based digital tasks such as filling in the same information in multiple places, re-entering data, or copying and pasting. In achieving this, the technology does not use actual ‘robots’ in the traditional sense, but rather a concept based on metaphorical software robots or on artificial intelligence (or digital workers). In particular, RPA usage has been increased by manufacturing firms in order to minimize the risks associated with the global corona virus situation. Therefore, demand for robotic process automation is anticipated to elevate once the corona virus pandemic abates. Given RPA provides additional layers of efficiency and visibility into the data infrastructure or businesses, companies who fail to modernize are at risk of losing out on significant returns. Scalability and flexibility are often called out as the key characteristics that attract businesses.
Pre-built artificial intelligence provides a potential solution for businesses automation. For example, prebuilt models can be used to recognize contact information from business cards or for activities like processing invoices. Another application is with prebuilt AI for marketing, and a third is with contract management solutions.
When Automaton Goes Wrong
While there are many texts and case studies for when automaton goes right, there are fewer examples of cases when automation fails to deliver. This article sets out some situations when automation goes awry. Automation may deliver some short-term gains, but in the long-term it can be a counterproductive force. Some reasons for this are:
- Automation locks in a technology cycle everyone knows all too well. Current-generation tech is replaced by more advanced tech. On the scale of the introduction of automation, that is likely to be an overly complex and expensive cycle.
- Current automation will be truly obsolete by the time artificial intelligence (AI) becomes the working machinery of business. There is no specific time frame involved, but it stands that AI-based automation will be far more efficient. Current advantages will, therefore, become future short-term advantages or actual liabilities. The competitive edge of the present forms of automation will evaporate, and this now old tech will become a liability. That could leave businesses holding expensive, uncompetitive equipment and a supply chain that cannot compete.
- Simply replacing automation systems will definitely not be the whole story, either. The likelihood is that like so many forms of technology, automated systems will evolve and diversify, probably pretty quickly. That gives businesses more opportunities, perhaps, but it also creates a much longer shopping list. There will be casualties from this cycle. Many of the gung-ho tech companies of the past and the present have hit this stage and simply not survived. They took the top-tech approach and missed the market realities completely.
- The kind of capital required for maintaining an automation advantage is huge. New technologies are always more expensive, often bug-ridden, and require some time to come onstream. Some simply do not deliver, while others turn out to be much less valuable than their costs.
 In other words, while automation is an obvious asset, it is also an instantly predictable liability and risk from the moment it is introduced. A further issue relates to technological and organizational barriers between information, communication, and automation technology functions.
In other words, while automation is an obvious asset, it is also an instantly predictable liability and risk from the moment it is introduced. A further issue relates to technological and organizational barriers between information, communication, and automation technology functions.
Some company executives distrust automation, connection to employment, professions, customer satisfaction, and other rather basic issues. This can be summarized according to two broad reasons, which are:
- While most senior executives have claimed that automation would free people from mundane repetitive tasks, exactly how (and even why) that happens is very unclear. If a robot takes a person’s job, does that instantly qualify them for a fabulous new job? Of course not.
- Automated systems are machines. They break down. They do not work properly. They have software bugs. They can cause real problems in many different scenarios, from infrastructure to basic business systems operations. Everybody has experienced these problems in some form.
Nonetheless, the drivers for automation appear to be outweighing the counterpoints. It is often the case with marketing that people will accept things they distrust or even hate intensely if there is a clear value to them. Thus, a company wishing to sell an idea or a product that buyers dislike will have to prove its value.
The human element should not be forgotten. It is important to ensure the organization has the right talent base and skills. To understand future capability needs, the pharma company must invest in training high-potential employees, and invest in hiring employees with the new required skill sets (for instance, advanced data analytics) during early stages to enable faster scale-up.
Avoiding Automation Failures with Risk Assessment
Like all other aspects of pharmaceuticals, quality risk assessments should be performed, and the principles of quality risk management adopted (8). This is not least because some forms of failure could well emerge from the interactions of independently designed and implemented components. Specifically, with automation, the following risks should be accounted for within each automation project (9):
- Human intervention should be built into the process for high-risk activities. This requires tackling human error. In turn this leads to error risk reduction, which is associated foremost with human error and the ways to reduce the impact of human error on procedures and processes. With this approach, it is important that the people carrying out the assessment should understand the different types of failure and the factors that make them more or less likely to occur (10).
- Complexity increases when automation involves multiple systems.
- Risks may increase where automation seeks to deliver faster speeds.
- Appropriate testing and validation must be in place to verify they can execute processes within boundary conditions.
- A detection system, in the form of alerts, needs to be in place to signal to human operators that a parameter is out of range or out of tolerance.
- Adequate controls must be established around data sent into the system to detect any outliers.
- Stress testing should be undertaken as part of system qualification to establish controls that support intended outcomes.
For each stage of an automation project, the risk analysis process should:
- Identify the known or foreseeable factors or hazards that could pose risk to the project; that is, any risk factors that can impact a project can be organizational, financial, or technological.
- Estimate the risks associated with each factor. For example, what could happen if the factor occurred and what would be the impact on the project?
- Estimate the probability of the risk occurring.
This leads to risk evaluation. The evaluation process need not be complex; instead the aim is to answer the question at each stage: Does the risk need to be mitigated or not? Once the high-risk tasks have been highlighted, then it is possible to prepare plans and countermeasures to overcome the risk. Risk analysis is not a static process; as an automation project progresses, a body of information is collected. This will document how a project risk has been managed and how effective the approaches have been thus far (11).
Risk also needs to be considered across the system lifecycle, considering:
- project definition and start-up
- evaluation and selection of the proposed system
- implementation of the system.
 A structured approach for assessing the risks of automation includes the use of event tree analysis, which considers the consequences of potential hazardous situations and develops countermeasures to reduce such consequences. Event tree analysis is a top-down, logical modelling technique for both success and failure that explores responses through a single initiating event and lays a path for assessing probabilities of the outcomes and overall system analysis. The process steps are:
A structured approach for assessing the risks of automation includes the use of event tree analysis, which considers the consequences of potential hazardous situations and develops countermeasures to reduce such consequences. Event tree analysis is a top-down, logical modelling technique for both success and failure that explores responses through a single initiating event and lays a path for assessing probabilities of the outcomes and overall system analysis. The process steps are:
- Define the system: Define what needs to be involved or where to draw the boundaries.
- Identify the accident scenarios: Perform a system assessment to find hazards or accident scenarios within the system design.
- Identify the initiating events: Use a hazard analysis to define initiating events.
- Identify intermediate events: Identify countermeasures associated with the specific scenario.
- Build the event tree diagram.
- Obtain event failure probabilities: If the failure probability cannot be obtained, use fault tree analysis to calculate it.
- Identify the outcome risk: Calculate the overall probability of the event paths and determine the risk.
- Evaluate the outcome risk: Evaluate the risk of each path and determine its acceptability.
- Recommend corrective action: If the outcome risk of a path is not acceptable, develop design changes that change the risk.
- Document the event tree analysis: Document the entire process on the event tree diagrams and update for new information as needed.
A limitation of event tree analysis is that it addresses only one initiating event at a time; in addition, partial successes/failures may not be distinguishable.
There is also failure mode and effect analysis (FMEA), which checks that the potential failures of the control and automation system are not overlooked. Furthermore, reliability assessment can be used with analysis methods to study the bottlenecks in the design and to prioritize the countermeasures whereby the risk can be reduced to attain an acceptable level. A variety of risk assessment tools suitable for pharmaceutical and healthcare organizations are outlined by Sandle. It is also important to assess health and safety concerns by deploying HAZOP (HAZard and Operability) studies. This methodology can be used to study the causes of potential accidents and to examine the control actions suitable for providing protection against them, thereby reducing the probability of accidents. As well as targeting accident reduction, this tool can contribute to an improvement in production efficiency.
The optimal way to manage risks is through placing all automation tasks within the change control framework, from the point of project initiation (12).
Limited cases studies
Another issue is with the paucity of case studies pertaining to automation. Pharma needs to see success, and often smaller payers hang onto the coat tails of bigger players. In this context, there is an adage often used among pharmaceutical technology personnel that states, “Once you have seen one implementation, you have seen one implementation”.  An informatics research study is subject to the same design characteristic limitations inherent in any single site study, including institution-specific factors (such as size, staffing models, unique workflows, and concomitant use of other technologies), differences in outcome assessment methods or definition, and probabilities associated with type 1 or type 2 errors. Type 1 error, in statistical hypothesis testing, refers to the error caused by rejecting a null hypothesis when it is true. Type II error is the error that occurs when the null hypothesis is accepted when it is not true. Type I error is equivalent to false positive (13).
An informatics research study is subject to the same design characteristic limitations inherent in any single site study, including institution-specific factors (such as size, staffing models, unique workflows, and concomitant use of other technologies), differences in outcome assessment methods or definition, and probabilities associated with type 1 or type 2 errors. Type 1 error, in statistical hypothesis testing, refers to the error caused by rejecting a null hypothesis when it is true. Type II error is the error that occurs when the null hypothesis is accepted when it is not true. Type I error is equivalent to false positive (13).
Summary
Although automation can herald many business advantages, as this article has pointed out, simply automating something does not create a process. Furthermore, if automation does not fit the context of the operation, it can sometimes prove to be a detriment. To ensure that the correct functions are targeted for automation and to avoid pitfalls when undertaking automation, using the guidelines and tools applicable to quality risk management provides a considerable advantage.
References
- ISA (2020) What is Automation?, International Society of Automation, at: https://www.isa.org/about-isa/what-is-automation
- Automation Concepts in Pharmaceutical Production. Pharmaceutical Online, at: https://www.pharmaceuticalonline.com/doc/automation-concepts-in-pharmaceutical-production-0001
- Wallis, P. (2019) Automation – CEOs love it, but there’s a Catch 22 and 23, Digital Journal, 11th July 2019, at: http://www.digitaljournal.com/tech-and-science/technology/op-ed-automation-ceos-love-it-but-there-s-a-catch-22-and-23/article/553802#ixzz6Tz0WWTut
- Parasuraman, R. and Riley, V. (1997). Humans and automation: Use, misuse, disuse, abuse. Human Factors, 39, 230–253
- Jämsä-Jounela, S-L. (2007) Future trends is process automation, IFAC Proceedings Volumes, 40 (1): 1-10
- Sandle, T. (2021) Big growth in robotic process automation forecast in 2021, Digital Journal, January 13th 2021 at: http://www.digitaljournal.com/business/big-growth-in-robotic-process-automation-forecast-in-2021/article/583854#ixzz6mZZRYNyy
- Donabedian A. (1968) The evaluation of medical care programs. Bull NY Acad Med. 44(2):117-124
- Sandle, T. (2016) Risk Assessment and Management for Healthcare Manufacturing: Practical Tips and Case Studies, DHI/PDA Books, River Grove, IL., USA

- Arntz, M., Gregory, T. Zierahna, U. (2017) Revisiting the risk of automation, Economics Letters, 159: 157-160
- Sandle, T. (2020) Error Risk Reduction: Concept and Case Study, Journal of Validation Technology, 26 (6) at: https://www.ivtnetwork.com/article/error-risk-reduction-concept-and-case-study
- Ahmed, A., Kayis, B. and Amornsawadwatana, S. (2007) A review of techniques for risk management in projects, Benchmarking: An International Journal, 14 (1): 22-36
- Hong, E-S; In-Mo L.; Hee-Soon, S.; Seok-Woo N.; Jung-Sik K. (2009). Quantitative risk evaluation based on event tree analysis technique: Application to the design of shield TBM. Tunneling and Underground Space Technology. 24 (3): 269–277. doi:10.1016/j.tust.2008.09.004
- Neyman, J.; Pearson, E. S. (30 October 1933). “The testing of statistical hypotheses in relation to probabilities a priori”. Mathematical Proceedings of the Cambridge Philosophical Society. 29 (4): 492–510
