Line integration - A first step to MES solutions
Standard process to execute line integration solution
In a running plant, modification and up-gradation comes with challenges like:
# Multiple automation platforms
# Ensure proper validation documentation
# In-line up-gradation and production plan together
# Unavailability of program backup
# Unavailability of modification details done in past
# Obsolete machines
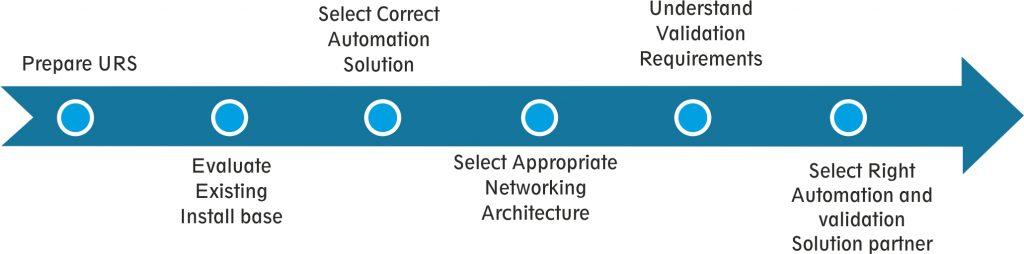
In a running plant, putting automation to achieve high level of data availability to ensure data integrity, along with production schedule, requires proper project management and strict scheduling.
User Requirement Specification (URS) and Install-base Evaluation
An elaborated URS and detailed plant study by skilled automation solution provider can help to segregate installed base into various categories which will help to make project management smooth.
Selecting appropriate Automation Solution
In recent years, open communication protocols help to take raw data from various devices and gives flexibility, scalability and future ready communication medium. Ethernet/IP is one of the open protocols which are compatible with plant IT network with a high level of data security.
There are majorly three different automation solutions available:
1. Integrate
2. Upgrade
3. Retrofit
Integrate
Usually the machines that fall in this category already come with open Ethernet connectivity. Just need to “integrate” these into central SCADA system. The machines have capability to communicate and give raw data with the open protocol, which can be easily accepted by standard data capturing software like SCADA or historian. This SCADA system will ensure batch reporting, audit trails, trending and activity log viewer for set point changes.
Upgrade
Machines with PLC and HMI which do not have open communication protocol, falls in this category. These machines need to be studied, in depth and each machine should be evaluated separately. Each machine should be replaced or upgraded with suitable communication hardware to make it compatible. As manufacturing plants have a variety of PLCs, the automation solution design should be compatible to all platforms. Also the solution provider should have ample knowledge or hands-on experience of such types of system.
Retrofit
These old/legacy machines are without PLC or with dedicated controller, which do not have standard communication protocol. These machines are mostly manual machines, but data which is available in these machines are important. The automation solution provider must have process knowledge or experience in similar machines. After refurbishing, these machine also have functions like unique user ID and password, audit trails, batch printout etc. Manual blenders are a classic example of such type of a system.
Validation Requirement
Pharmaceutical industry is driven by various guidelines for validation and documentation. Up-gradation process should have proper documented evidence to provide during various audits. As per the latest guideline of GAMP 5, Risk assessment documents are also required. Each category requires separate set of documents. Few of them are listed below:
a) User Requirement Specification (URS)
b) Functional Design Specification (FDS)
c) Functional Risk Assessment (FRA)
d) Installation Qualification (IQ)
e) Operation Qualification (OQ)
f) Validation Summary Report (VSR)
Selecting Right Automation and Validation Partner
Selecting an appropriate automation solution provider for up-gradation or retrofitting is very important in such projects. A few capabilities of a solution provider are mentioned below, which may speed-up the process:
i) Enough knowledge of process as well as control strategies.
ii) Able to work on multiple automation platform.
iii) Capable to design solutions for each category.
iv) Having knowledge of industrial networking.
v) Able to do/support on validation and documentation activity.
Kevin’s Solutions
- Automation Solutions:
1.1 Up-gradation of machines to collect data on a common open protocol.
1.2 Integration of Process Equipment.
1.3 Retrofitting of existing systems.
1.4 Integration of utilities.
1.5 Device Level Redundancy Networking.
1.6 Audit Trail / Named User ID functionalities for 21 CFR part 11.
1.7 Compliance of HMI’s.
- Regulatory compliance solutions
- 21 CFR part 11/EU Annex 11 assessment.
- Comprehensive GAP Assessment
This article is contributed by Prachetas Mehta, Sr. Manager – S & M, Kevin Technologies, Ahmedabad, India.

