Microbiological Contamination Risks in Cleanrooms
Key Insights and Best Practices

 By Dr Tarun Chugh
By Dr Tarun Chugh
Microbiological contamination is a critical concern in pharmaceutical manufacturing, particularly for drug products purporting to be sterile. Cleanrooms serve as the backbone of contamination control strategies, yet they often fall short due to deficiencies in design, maintenance, and operational practices. Regulatory bodies such as the US Food and Drug Administration (FDA) consistently highlight such issues in their observations and warning letters. This article delves into the common deficiencies cited under 21 CFR 211.113(b), examines their implications, and outlines best practices for maintaining cleanroom compliance.
Key Deficiencies in Cleanroom Design and Maintenance
1. Lack of Written Procedures
 Written procedures serve as the foundation for consistent operations in controlled environments. Failure to establish and follow robust standard operating procedures (SOPs) can lead to variability in cleanroom practices, increasing the risk of microbial contamination. SOPs should address critical areas such as cleaning schedules, gowning protocols, and material flow to ensure uniformity and compliance.
Written procedures serve as the foundation for consistent operations in controlled environments. Failure to establish and follow robust standard operating procedures (SOPs) can lead to variability in cleanroom practices, increasing the risk of microbial contamination. SOPs should address critical areas such as cleaning schedules, gowning protocols, and material flow to ensure uniformity and compliance.
2. Inadequate Environmental Monitoring
Environmental monitoring (EM) is essential to ensure that cleanroom conditions remain within acceptable limits. Common lapses include insufficient sampling frequency, improper selection of sampling locations, and failure to monitor high-risk areas. An effective EM program involves proactive monitoring of air, surfaces, and personnel to detect microbial excursions early and take corrective actions promptly.
3. Deficiencies in Cleaning and Disinfection Practices
Effective cleaning and disinfection are paramount to maintaining a contamination-free environment. Regulatory observations frequently cite issues such as inadequate cleaning protocols, improper use of disinfectants, and reliance on single-agent disinfectants without rotation. Incorporating validation studies and periodic reviews of cleaning practices can mitigate these risks.
4. Poor Personnel Practices
Personnel are a significant source of contamination in cleanrooms. Non-compliance with gowning protocols, poor hygiene, and lack of aseptic training contribute to contamination risks. Regular training programs and stringent adherence to gowning procedures are essential to minimize human error in controlled environments.
5. Contaminated Drug Products
Microbial contamination of drug products intended to be sterile is a severe regulatory and patient safety concern. Such incidents often stem from inadequate sterility assurance measures, improper handling, or equipment-related contamination. Adopting robust sterilization processes and implementing stringent controls can safeguard product integrity.
6. Inadequate Validation of Aseptic Processing
Process validation is critical to demonstrating that aseptic processes consistently produce sterile products. Common deficiencies include incomplete media fill studies, failure to simulate worst-case conditions, and inadequate documentation. Comprehensive validation protocols that account for operational variability can strengthen sterility assurance.
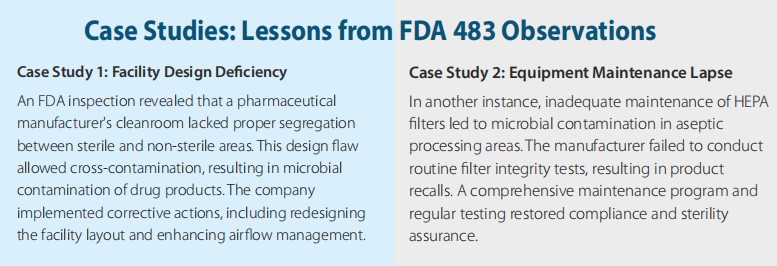
7. Equipment Maintenance and Cleaning Failures
 Equipment used in sterile manufacturing must be meticulously maintained and cleaned. Deficiencies in equipment maintenance, such as neglected filter replacements or inadequate cleaning of tanks, contribute to contamination risks. Implementing preventive maintenance schedules and validating cleaning procedures are vital steps.
Equipment used in sterile manufacturing must be meticulously maintained and cleaned. Deficiencies in equipment maintenance, such as neglected filter replacements or inadequate cleaning of tanks, contribute to contamination risks. Implementing preventive maintenance schedules and validating cleaning procedures are vital steps.
8. Deficiencies in HEPA Filter Integrity
High-Efficiency Particulate Air (HEPA) filters are critical for maintaining cleanroom air quality. Regulatory observations frequently highlight failures to test HEPA filter integrity or address leaks. Routine testing, coupled with regular inspections, ensures the effectiveness of air filtration systems in controlled environments.
9. Inadequate Design of Facilities
Facility design plays a pivotal role in contamination control. Poor layouts, improper segregation of high-risk areas, and insufficient airflow management can lead to cross-contamination. Regulatory guidelines emphasize the importance of designing facilities that support unidirectional airflow, effective segregation, and easy maintenance.
10. Gaps in Investigation of Contamination
 Thorough investigations of contamination incidents are critical to identifying root causes and preventing recurrence. Common gaps include insufficient data analysis, lack of trend reviews, and failure to implement effective corrective actions. A systematic approach to root cause analysis, supported by tools such as Fishbone diagrams, can enhance contamination investigations.
Thorough investigations of contamination incidents are critical to identifying root causes and preventing recurrence. Common gaps include insufficient data analysis, lack of trend reviews, and failure to implement effective corrective actions. A systematic approach to root cause analysis, supported by tools such as Fishbone diagrams, can enhance contamination investigations.
Best Practices for Cleanroom Compliance
To address these deficiencies and maintain compliance with 21 CFR 211.113(b), manufacturers should adopt the following best practices:
1. Develop Comprehensive SOPs: Ensure that all cleanroom activities are governed by detailed, clear, and well-documented SOPs. Regularly review and update these procedures to align with regulatory requirements and technological advancements.
2. Strengthen Environmental Monitoring Programs: Implement risk-based EM strategies, including frequent sampling in critical areas. Use trend analysis to identify patterns and proactively address potential issues.
3. Enhance Cleaning and Disinfection Protocols: Validate cleaning and disinfection procedures to ensure their effectiveness. Rotate disinfectants and incorporate sporicidal agents to prevent resistance.
4. Invest in Personnel Training: Conduct regular training sessions on aseptic techniques, gowning protocols, and cleanroom behaviour. Reinforce a culture of accountability and adherence to procedures.
5. Validate Aseptic Processes: Perform comprehensive validation studies, including media fills under worst-case conditions. Maintain thorough documentation of validation results and continuously monitor process performance.
6. Optimize Facility Design: Design cleanrooms to support contamination control, with unidirectional airflow, effective segregation, and easy maintenance. Collaborate with experts during the design phase to ensure compliance.
7. Maintain Equipment and Systems: Implement preventive maintenance programs for all critical equipment. Validate cleaning processes and ensure that HEPA filters are tested and replaced as needed.
8. Conduct Robust Investigations: Develop a systematic approach to investigating contamination incidents. Use root cause analysis tools to identify issues and implement effective corrective and preventive actions (CAPA).
Conclusion
Maintaining cleanroom compliance is critical to ensuring the microbiological quality of drug products. By addressing common deficiencies and adopting best practices, pharmaceutical manufacturers can mitigate contamination risks, meet regulatory expectations, and safeguard patient health. Continuous improvement and proactive management of cleanroom operations are essential for achieving long-term compliance and operational excellence.
References
1. U.S. Food and Drug Administration. “Code of Federal Regulations Title 21, Part 211.113(b).”
2. International Organization for Standardization. “ISO 14644: Cleanrooms and Associated Controlled Environments.”
3. FDA Warning Letters Database. “Examples of 483 Observations.” Retrieved from FDA.gov.
4. Parenteral Drug Association. “Technical Report No. 13: Fundamentals of an Environmental Monitoring Program.”
5. ISPE Baseline Guide. “Volume 3: Sterile Product Manufacturing Facilities.”
About The Author
Dr. Tarun Chugh has 30+ years experience in pharma industry with and performing consultancy (SIMco Pharma Consultancy) for Audits, Compliance, Training, Regulatory, Coaching and guidance for Parenteral, Microbiology, Oral Dosage, APIs, Protocols, SOPs, Documents Review, Risk Assessment and Lean Six Sigma. He has done Doctorate in Microbiology and has good exposure in environmental
monitoring as per regulatory requirements.
Exclusive
IPA’s 10th Global Pharmaceutical Quality Summit
Articles
Key Insights and Best Practices By Dr. Tarun Chugh
A Seasoned Perspective By Manan Dave
By Todd Martin, Natoli Engineering Company, Inc
Infocus
Presented by UhImann India, Optima, and Glat
Kevin Process Technologies
Tests and Process Optimization in Hyderabad





