Top Five Priorities
To mitigate Regulatory Challenges in Aseptic Manufacturing
 By Dr. Subrata Chakraborty
By Dr. Subrata Chakraborty
Aseptic products are increasingly taking centrestage in pharmaceutical manufacturing as more and more high value complex formulations, biopharmaceuticals and advanced therapy medicinal products (ATMPs) are adding to the treatment options for critical ailments. The objective of this article is to highlight the most critical aspects to manage product quality and patient safety and in turn ensuring business continuity.
According to a 2022 McKinsey report, the global sterile manufacturing market is expected to grow by over 50% by 2028. However, this segment of business also suffers a major business continuity risk, primarily driven by regulatory compliance challenges. Any compliance issue can potentially trigger significant impact, ranging from approval delays and market recalls to hefty legal penalties. All of these can jeopardize a company’s operations, financial health, and reputation. In an ever-evolving regulatory landscape, where patient safety is paramount, and guidelines are growing increasingly stringent and prescriptive, the aseptic product businesses are constantly challenged with compliance issues that disrupt their business continuity plans.
 Unlike other dosage forms, aseptic manufacturing requires substantial capital investment and high operational costs. Setting up a sterile production line is a lengthy process, which typically takes about 2 to 4 years from concept to commercialization. This extended timeline is due to factors like long equipment lead times, complex validation procedures, and the necessity for regulatory approvals, all of which drive up the business stakes. As a result, business owners are continually seeking ways to safeguard their investments.
Unlike other dosage forms, aseptic manufacturing requires substantial capital investment and high operational costs. Setting up a sterile production line is a lengthy process, which typically takes about 2 to 4 years from concept to commercialization. This extended timeline is due to factors like long equipment lead times, complex validation procedures, and the necessity for regulatory approvals, all of which drive up the business stakes. As a result, business owners are continually seeking ways to safeguard their investments.
The pressing question remains: How can uncertainties and the risk of failures be minimized?
There is no easy answer to this and no quick fix. The lack of sterility or assurance of sterility continues to be the most common reason for pharmaceutical product recalls.
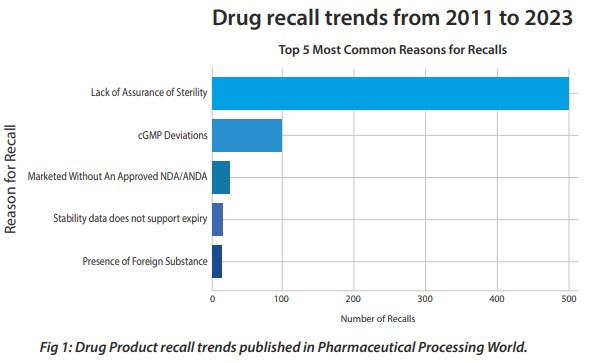
Unlike a terminally sterilized product where the sterility assurance levels can be mathematically calculated and validated, aseptic products’ sterility assurance depends on several factors. It starts from facility and equipment design to environmental controls, materials and components control, operators’ behaviors, input materials sterilization, end products testing, so on and so forth. Sterility testing is one of the controls for assurance, but it alone cannot guarantee a sterile product. Hence in this case product quality and patient safety depends on meticulous controls in each of these areas.
Objective of this article is to highlight the most critical aspects to manage product quality and patient safety and in turn ensuring business continuity.
1. Quality Culture: walk the talk
This is fundamental to achieving consistent quality outcomes and mitigating business continuity risks in a regulated pharma business. A true quality culture is one that infiltrates every level of an organization, beginning at the top and flowing down through every employee and decision-making process.
Leadership plays a critical role in setting the tone for quality. One poor decision or action by a top leader can set a precedent for routine compromises, spreading like wildfire throughout the organization. Conversely, when leaders demonstrate an unwavering commitment to quality, it sets a powerful example that resonates across all levels.
For instance, I have seen companies make tough decisions like rejecting entire campaign of batches worth millions of dollars because they were on the borderline of quality standards. While such decisions may not always seem financially prudent, they are in fact, investments in the organization’s long-term health and reputation, setting a high standard for quality within the company.
 Quality culture is not about ticking boxes on a quality matrix, nor is it about making great presentations in quality review meetings or launching initiatives with catchy names. It’s about embedding quality into the very fabric of the organization. This begins with the recruitment and onboarding of employees and extends to the selection and qualification of equipment and machines, ensuring they meet the highest standards before they are put into operation, rigorously qualifying materials and vendors, and tight control over release testing to ensure that every input into the production process meets rigorous standards.
Quality culture is not about ticking boxes on a quality matrix, nor is it about making great presentations in quality review meetings or launching initiatives with catchy names. It’s about embedding quality into the very fabric of the organization. This begins with the recruitment and onboarding of employees and extends to the selection and qualification of equipment and machines, ensuring they meet the highest standards before they are put into operation, rigorously qualifying materials and vendors, and tight control over release testing to ensure that every input into the production process meets rigorous standards.
Building a strong Quality Culture requires organization’s commitment to surpass several challenges. Firstly, it necessitates a fundamental shift in mindset and behavior across the organization. This requires overcoming resistance to change and embedding quality deeply into the organization’s core values and practices. Another challenge lies in ensuring active participation and commitment from all employees.
Cultivating a Quality Culture demands that everyone in the organization is engaged and well-informed. This means investing in ongoing training, effective communication, and continuous education programs to provide employees with the knowledge and skills they need to uphold quality standards. Lastly, establishing a Quality Culture often involves considerable investment in technology, infrastructure, and training. Allocating the necessary resources to support these quality initiatives can be a significant financial hurdle, particularly for smaller organizations that may struggle with tighter budgets.
2. Contamination Control Strategy (CCS) - Beyond Compliance
Since the new Annex-1 became effective, there is a huge wave of discussions and deliberations to implement CCS. This regulatory update has highlighted the need for sterile manufacturing sites to adopt a more robust and comprehensive approach to contamination control. However, inspection outcomes reveal a significant gap between these discussions and their practical implementation.
The main challenge isn’t just drafting a document called CCS; it’s about making sure the strategy is truly comprehensive and effective at both the unit and site levels. The CCS should be more than a superficial effort—it needs to be built based on the accumulated knowledge of the organization’s systems and processes. It’s important to recognize that a CCS isn’t just another document for regulatory audits. Instead, it should be a core strategy integrated into every part of the manufacturing process, helping the team stay aware of contamination risks and how to control them.
A well-designed CCS must integrate all facets of the manufacturing process, from facility design and equipment controls to personnel practices, environmental monitoring, and stringent control over materials. This integration requires a cultural shift within the organization, moving from reactive contamination correction to proactive contamination prevention. This means that contamination control is not merely about responding to issues as they arise but preventing them from occurring in the first place.
Further, a CCS is not a static document; it is a living strategy that must be continuously reviewed, updated, and embedded in everyday operations. The success of a CCS depends on its adaptability and the commitment of the entire organization to uphold its principles.
 Building a CCS is not the responsibility of a single person or department. It requires a collaborative effort from a cross-functional team that includes subject matter experts from various areas of the organization. This team should be led by a competent leader who has been granted the necessary authority to drive the implementation of the CCS. In many cases, it is also beneficial to seek external expertise to provide an independent perspective and to train the internal team on best practices in contamination control. External experts with sufficient experience can offer valuable insights and ensure that the CCS is both comprehensive and effective.
Building a CCS is not the responsibility of a single person or department. It requires a collaborative effort from a cross-functional team that includes subject matter experts from various areas of the organization. This team should be led by a competent leader who has been granted the necessary authority to drive the implementation of the CCS. In many cases, it is also beneficial to seek external expertise to provide an independent perspective and to train the internal team on best practices in contamination control. External experts with sufficient experience can offer valuable insights and ensure that the CCS is both comprehensive and effective.
3. Keep People away from the process
It’s a well-known fact that humans are one of the most significant sources of contamination in cleanrooms, primarily due to shedding skin cells, hair, and other particulates. Even with proper gowning and hygiene practices, the presence of personnel in critical areas can introduce contaminants. By use of technologies that minimize human presence in grade A environments, such as using isolators, barrier technologies or robotics, manufacturers can significantly reduce the risk of contamination. This not only protects the drug products from contamination but also ensures a more controlled and cleaner environment, which is crucial for maintaining high-quality standards in aseptic manufacturing.
 Human involvement in manufacturing processes also introduce variability due to differences in skill levels, experience, and fatigue. In aseptic processing, where need for maintaining each unit contamination free is paramount, even minor inconsistencies can lead to contamination risks. Automation and advanced technology ensure that processes are performed with uniform precision, reducing the likelihood of errors and enhancing the overall reliability of production.
Human involvement in manufacturing processes also introduce variability due to differences in skill levels, experience, and fatigue. In aseptic processing, where need for maintaining each unit contamination free is paramount, even minor inconsistencies can lead to contamination risks. Automation and advanced technology ensure that processes are performed with uniform precision, reducing the likelihood of errors and enhancing the overall reliability of production.
No human intervention is safe in aseptic processing environment. Minimizing human intervention reduces the chances of human error and hence, the risk of contamination. Automated systems can be designed to consistently meet regulatory requirements, ensuring high levels of sterility and product safety.
Automated systems can operate continuously, without the need for breaks or shift changes, leading to higher productivity. They can perform repetitive and complex tasks more quickly and accurately than humans, streamlining the production process. This not only increases output but also reduces the risk of contamination.
4. Invest in Good Training
The importance of effective training in aseptic manufacturing and testing cannot be emphasized enough. Many of the recent regulatory citations directly or indirectly questions the effectiveness of training provided to shopfloor personnel. This trend suggests that training is often viewed as a mere checkbox item in the list of compliance activities, rather than as a vital component of compliance and operational excellence.
In some organizations, it’s common for new employees to be overwhelmed with a large stack of SOPs that they must train on and qualify for.  Managers often prefer to train everyone in the department on every SOP, aiming to create a flexible workforce capable of covering for any absent colleague. However, this approach prevents the genuine development of employees, ensuring they are truly competent for their specific tasks.
Managers often prefer to train everyone in the department on every SOP, aiming to create a flexible workforce capable of covering for any absent colleague. However, this approach prevents the genuine development of employees, ensuring they are truly competent for their specific tasks.
Key questions that need to be asked to evaluate the effectiveness of current training practices are:
Are your trainers truly qualified? It’s not enough for trainers to be subject matter experts. Effective training requires more than subject knowledge; it demands the ability to communicate knowledge clearly and engage learners. Not every expert is a skilled trainer, and recognizing this distinction is crucial.
Is training tailored to individual needs? Training should be based on an assessment of each employee’s current knowledge and skills, not just on what managers believe they need to know. It’s important to understand what and how much a trainee is capable of retaining. A personalized approach ensures that training is relevant and effective.
Does training focus on skill building? While knowledge of SOPs is essential, practical, on-the-job skills are equally important. Do you have mechanisms in place to ensure operators receive skill trainings to perform the critical aseptic manipulations? Do you evaluate and rate their critical skills before they are assigned a job role?
Is there a feedback mechanism from daily performance? Regular performance feedback by competent observers can be a good complement to the overall training and development program of aseptic personnel.
How many times you need to retrain employees as a CAPA? If there are frequent instances where you have the reason for failure as human error, and you retrain the operator – it indicates either the investigation is incomplete, or the training was not effective at the first place.
These questions underscore the need for a shift in how we approach training in aseptic manufacturing. Investing in comprehensive, well-designed training programs is not just about meeting regulatory requirements—it’s about building a workforce that is truly prepared to maintain the highest standards of quality and safety in the production environment.
5. Scientifically Sound Process Design
In aseptic manufacturing, where the probability of detecting product contamination is extremely low, and margins for error are minimal, ensuring product safety hinges on the strength of the process design. Even with investments in high end automations and the latest barrier technologies, a poorly designed process can still breach the aseptic conditions and increase the risk of contamination. Further, a poor process design can never be adequately compensated by procedural controls, no matter how stringent they are. This is why process design must be meticulously crafted from the earliest stages of product development, continuing through technology transfer and scale-up phases.
For instance, in complex formulations involving aseptic compounding, the addition of sterile materials or sampling activities can introduce significant contamination risks unless they are performed in a fully enclosed, Grade A environment. Similarly, the transfer of machine parts post-sterilization to the filling RABS (Restricted Access Barrier System), or the handling of pre-sterilized components such as media plates and cleanroom consumables, can pose serious contamination threats if not managed properly. These risks highlight the importance of designing a process that is inherently robust and built on a foundation of risk assessment.
A well-designed process should integrate the right technologies, calibrated for their specific applications, to ensure that every step—from R&D to production—is optimized for contamination control. The focus should be on creating a closed and controlled environment where human intervention is minimized, and the likelihood of contamination is drastically reduced.
Hence, the experts who are involved in product design and technology transfers should have good understanding of the aseptic processes apart from the product chemistry. This risk-based approach not only enhances product safety but also ensures compliance with regulatory standards, ultimately leading to a more reliable and efficient manufacturing process.
In conclusion, the path to achieving excellence in aseptic manufacturing is paved with strategic investments in process design, robust contamination control strategies, and a strong quality culture. As the landscape of regulatory expectations continues to evolve, particularly with the new Annex 1 guidelines, it has become increasingly clear that a superficial approach to compliance is insufficient.
Building a holistic Contamination Control Strategy (CCS) is not merely about ticking boxes; it’s about embedding a proactive, risk-based mindset into every facet of the manufacturing process. This approach must permeate every level of the organization, from top leadership down to the shop floor, promoting a culture that prioritizes quality above all else.
Moreover, investing in technology-enabled process design is not just a necessity but a safeguard against the inherent risks of aseptic manufacturing. A poorly designed process, reliant on human intervention and subject to procedural controls, is vulnerable to failure. Instead, by leveraging advanced technologies and collaborating closely with cross-functional teams and external experts, manufacturers can create processes that minimize contamination risks and enhance product safety.
 Ultimately, the future of aseptic manufacturing lies in a commitment to continuous improvement, a dedication to robust training, and an unwavering focus on quality. By adopting these principles, manufacturers can not only meet regulatory standards but also drive innovation and ensure the delivery of safe, effective products to patients.
Ultimately, the future of aseptic manufacturing lies in a commitment to continuous improvement, a dedication to robust training, and an unwavering focus on quality. By adopting these principles, manufacturers can not only meet regulatory standards but also drive innovation and ensure the delivery of safe, effective products to patients.
About the Author
Dr. Subrata Chakraborty, the Founder and CEO of INOVR, a Virtual Reality-based educational platform, designed exclusively for the pharmaceutical industry. With 27+ years in Manufacturing, Quality operations, Validations, and Training, he’s an expert in Aseptic Processing and contamination controls. Beyond his SME role, Subrata significantly impacted people and organization development during his decade-plus leadership roles at Pfizer, , Novartis, Fresenius-Kabi, and Cipla. Recognized for his expertise, he actively contributes as an author, speaker, and technical
reviewer in global industry forums like PDA, ISPE, and SfSAP. Subrata’s multifaceted career exemplifies education and industry standards.
Parle Exclusive
Articles
A Lean Approach, by Navdeep Singh Kathuria
To mitigate Regulatory Challenges in Aseptic Manufacturing, by Dr. Subrata Chakraborty
Thoughts on Sustainability Drives by Prabir K Das
















