Onsite Factory Acceptance Test Vs Virtual Factory Acceptance Test
Based on survey report on “Virtual Factory Acceptance Test” in pharmaceutical industry
Digitization is an improved way to simplify our life and the same applies to manufacturing industries. Digitization is an enhanced, simplified, cost-saving, productive and most effective tool for developing the industries to meet world standards. In this race, the pharmaceutical sector moved a step forward to digitize the industry through Virtual or Remote FAT.
The Coronavirus pandemic cannot stop us and our projects from being completed on time. It made industry to find a new way to maintain the relation between OEMs and life science sectors.

by Mr. Varadharaj Vijaykumar
The restriction for traveling from place to place was implemented during lockdown to prevent the spread of the virus. Pharmaceutical companies found a new way to complete the projects from their workplace. Virtual FAT is a part of it. This addendum to the existing Factory Acceptance Test (FAT) work product tackles how to most effectively use communication and connection technologies to allow a life science manufacturer to interact remotely for their newly purchased equipment/system with original equipment manufacturers (OEMs), via live stream or a video conferencing facility.
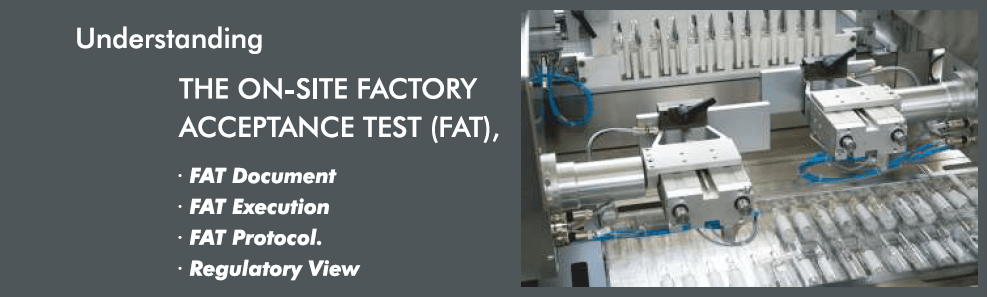
Factory Acceptance Test (FAT):
Is a process, evaluating the equipments during and after the assembly process by verifying that it is built and operating under design specifications as per purchase order. A Factory Acceptance Test (FAT) is usually preformed at the vendor prior to shipping to a customer. The supplier tests the machine/system in accordance with the clients approved test plans /purchase orders and specifications to show that system is at a point to be installed and tested on receivers site.
The FAT Document:
Factory Acceptance Testing is an inspection that incorporates both static and active comprehensive testing of framework or significant framework segments to help the capability of the system. The tests must verify that the system meets the design and functional specification mentioned in the User Requirements Specification (URS) is embodied and performs as specified as per the documents. It is written by the manufacturers and executed by the client or by the client’s project team.
Both parties can be assured that the equipment meets all the contractual specifications. It also provides an opportunity to make corrections or adjustments for any issues before shipping to the client site.
Execution of FAT:
Factory Acceptance Testing is executed at the vendor’s test facility. The FAT document must be written to fully challenge the Functional Specification (FS) that was derived originally from the User Requirements Specification (URS). Regulatory compliance standards must be used for writing the FAT. Depth Knowledge is required to write the FAT Protocol. The client send some members of project at the vendor site for conducting tests and also to get trained on the machine.
Who should write FAT Protocol?
Vendor shall write this protocol and same shall be reviewed by site team as they can recommend additional tests and same shall be included in FAT protocol.
Regulatory view on FAT:
In the Pharmaceutical, Biotech and Medical device industries, FAT documentation are routinely used to ensure that is compiled all GMP requirements that are legislated mainly through CFR Parts11/210/211/820 in the USA and in similar legislation throughout the globe. As per EU Annex 15: Qualification and Validations regarding FAT
“Equipment, especially if incorporating novel or complex technology, may be evaluated, if applicable, at the vendor prior to delivery”.
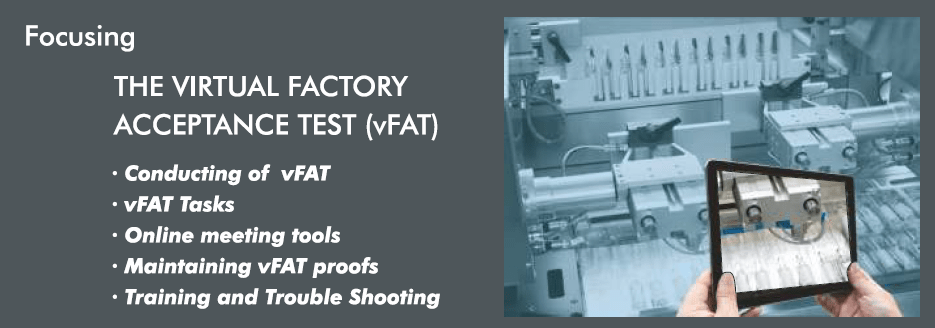
What is Virtual Factory Acceptance Test?
The vFAT is based on a virtual way of conducting tests for the equipment/system at the vendor site. Simply, where the customer can avoid travelling thousands of kilometres thus save travel time to watch their live running machines. With Virtual FAT, the client can watch the tube or closure running of the equipment/system. They can inspect the critical parts of the live running machines and can discuss important things with the vendor from wherever he, she, or they may like.
Conducting of Virtual FAT OEM uses multiple high-quality cameras to give the best possible view of the machine. One camera is placed to view the entire area. Other cameras are placed around the device to see specific details. The control panel can be watched live with the movable camera to get the result, data, performance and functioning of the machine. The manufacturers are flexible to move each camera according to the customer’s needs or specific requests.
Requirements for the online meeting tools:
High-speed internet connects plays a vital role for vFAT, the other requirement like a laptop, a computer, a tablet, or a mobile phone equipped with a microphone and speaker. The headset gives better audio quality than using portable speakers. There is no requirement for any specific software. However, some vendors take the support of online tools like WebEx, Zoom, Google Meet and Microsoft teams. The meeting is conducted by sharing a link of these online tools by the manufacturer.
Document Handling:
Vendor should have a controlled copy of the FAT issued via Document Control or by SCAN copies after approval by the site QA team.
Lastly, a signature log of the vendor and site team to witness their presence physically equipped to execute the FAT (live video play-back, handheld cameras and daily log sheet etc.)
Timelines:
Before this pandemic, the timelines for conducting onsite FAT can be pre-planned and fixed by the FAT team. But in current situation, timelines gets exceeded due to virtual FAT, which takes place virtually. Time-consuming happens from both the vendor and the site team. It can take 2-3 hours a day to set-up the live streaming and ensure that both the audio and visual parts are working correctly and all online documents are verified.
Cost and Expenditure:
As the site team conducts FAT from their premises, the client saves the high travel and accommodation expenses. The client also saves the travel time of the team, which can be a week or more? Generation of a document, time, internet connections, and accessories can cost a bit from both vendor and project team.
Due to virtual FAT, purchase order needs to be balanced from both manufacturer and site team, which is different from the original project scope (Initial costings may differ).
Using Virtual Smart Glasses (Optional)
The on-site manufacturer can use the Virtual Smart Glasses, these glasses make the instructor hands-free while moving around the environment and projecting their view of instruments, equipment, and more to the rest of the team. This type of platform is also interactive, this allows the instructor to see and talk with all participants and also help to pull documents into smart glasses, while still seeing their environment and making the smooth operation for Virtual Factory Acceptance Test.
Virtual Machine Training & Trouble Shooting:
It is vital to train the operator. Hence live virtual machine training with training modules and multiple training sessions is mandatory for the smooth operation of the machine. There should be a service team that can perform troubleshooting with virtual access to resolve the issues and give smooth function of equipment.
What do Pharma companies is expected to do in line with OEM for Conducting Virtual FAT:
All this virtual/Virtual FAT Protocols are to be pre-approved by quality assurance team, and detailed plan to be made for smooth functioning, so that the presence of QA for witnessing the “real time” FAT. In order to make this, change control to be raised along with risk assessment, detailing the procedure and risks involved while conducting the Virtual FAT.
Standard operating Procedures should be in place for Virtual FAT:
The quality Assurance team shall have a Quality Vendor Audit/Review of QMS for conducting Virtual FAT Procedures of Vendors. Assessment of vendor training on the Project pre-requisites procedures (FAT Plan, Punch list Plan, GDP Plan) by QA team.
Initial Training:
The site team is not trained or experienced with the Virtual FAT. Hence before beginning Virtual FAT, OEM will conduct initial training with the site team concern to the accessories and tools while handling. Training aspects should include technical capabilities, safety systems, IT systems, hardware and software to be used.
Daily Punch list:
The punch listing for Onsite FAT and Virtual FAT will remain the same. The OEMs and the project team should follow the same approved procedure.
Project Review:
A close review of project documents is required with the vendors to ensure a smooth Virtual FAT. Instead of dragging many things concern to purchase order, a close working relationship will help us for good and smooth functioning of Virtual FAT. Vendors should also have a continuous connection with the purchasing team needed with all mails or with telephonic discussions. Every minute of meetings for all talks are to be shared. The same has to be noted and documented.
What can be done for the FAT proofs?
On request, while performing the Virtual FAT the same can be recorded by both, client and the vendor, on completion, the digital recorded clips can be shared with each other to maintain the records. This will benefit both the parties in case of any disputes arise in future.
Limitations of Virtual FAT:
- Controls exist with the testing engineer.
- Internet connectivity between On-site test engineers and receiver site.
- Communication gap between engineer and receiver due to language barrier.
- Finally with time zone between different countries.
What will be missed in the Virtual FAT Apart from GMP?
At the maximum point, there will be the same experience in Virtual FAT as that of On-site FAT, like a live machine in action. What will we miss? At the most, there is a lack of interaction between experts from both the end, travelling to the workshop, onsite hospitality, personal meet and greet.