Survey Report on Virtual FAT Conducted in Pharmaceutical Industry
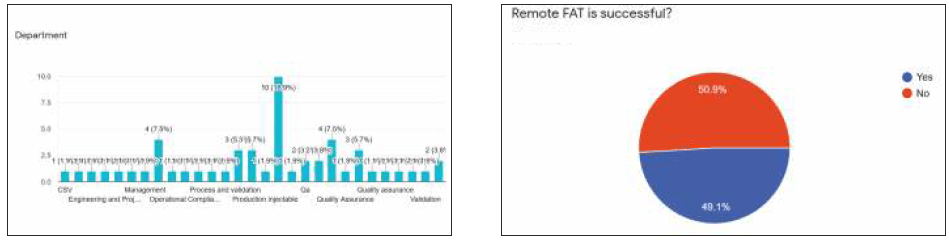
A survey was conducted on Virtual FAT (Factory Test Acceptance) in this COVID-19 pandemic. This survey has got an eminent response from an Indian pharmaceutical organization. Many delegates from the different departments/professionals, such as Quality, Engineering, Production, Validations, and Consultants participated in it.
The survey focused on the understanding of Virtual FAT concepts from the Indian pharma Industry. People from different experiences participated actively in this survey. 49.1% of people responded that they had accepted the vFAT considering this COVID-19 pandemic. Meanwhile, 50.9% of people concluded that it is not successful. However, their views on this topic are mention below.
The survey reported with pros and cons of vFAT
Pros:
- Via vFAT, the employee saves their travel time; they do not need to travel anywhere to inspect the machine
- Faster in terms of business
- vFAT is a cost saving; one can inspect the machine via phone through internet.
- No Pandemic pressure or threat on employees.
- FAT can be checked by Virtual recording whenever and anywhere as required.
Cons:
- The project team cannot see the running status of the machines closely.
- There is an ascertained for Physical Equipment and complete readiness cannot be ascertained.
- Human aspect, QMS of client and other real issue can be missed if protocol not good
- Some activities may not visible properly, Machine finishing issues may remain as it is.
- It is challenging to verify Performance, Turbulence, Noise, etc. through Virtual FAT.
- Operators and Engineers will miss the physical training, which OEMs used to conduct during Physical Onsite FAT.
Suggestions from the Participants:
- Due to pandemic, remote FAT can be acceptable. Once the pandemic ends, it needs to be back to onsite FAT.
- Usage of a high-quality camera during Remote FAT is needed.
- Depend on the machine design and critical part, decision to be taken to conduct virtually or onsite and also business urgency.
- Even in case of virtual FAT, there must be an agreement between user and vendor for any dissatisfaction. It must handle at the factory during installation (but not critical change).
- Documents should have a digital signature and thoroughly verified by the user before the dispatch of the machine. It is also required to build a team that verifies the videos of virtual FAT and submits their report to the leads of user and vendor.
- If any video conferencing done during virtual FAT, it should be part of the final report.
- Trained professionals to demonstrate the FAT against the URS. High-end cameras handling with voice recorder to be the new requirements, E-sign of documents is expected.
Conclusion:
Due to this ongoing pandemic, we all had an unplanned Virtual FAT process enforced upon us. However, as a part of project, all FAT Testing procedure had to summarize in the FAT Report with detailed punch list, daily discussions and Virtual meetings.
Looking at the above pros and cons for Virtual FAT, and also the suggestion from the delegates. We can conclude that effective measures should be taken and any open points or remarks during virtual FAT should be clear and completed as per the compliance before dispatching or at the receiver/client site.
Considering above points with appropriate execution in a traceable manner, will have greater effect on leveragability in tests and this will lead to success of Virtual FAT. But ensure it shall have minimal dis assembling of machine while dispatching.
About the Author:
Mr. Varadharaj Vijayakumar is an experienced pharma professional, diligent in his core functional area (pharmaceutical technical operations) and committed to the profession.
His main area of expertise is aseptic processing & areas of interests are sterility assurance, manufacturing technology, process validation, failure investigations, troubleshooting, Isolator Technology Cycle developments, Risk assessments/QMS Gap assessments, Lyophilization, Glove Integrity testing’s.
He has been involved in many projects from concept design to successful delivery of commercial product to the continuous improvement phases and also led organization(s) efforts with teams in managing audits and validations aspects
He is a postgraduate in Pharmaceutical Sciences and an active member of professional associations like PDA, IPA, Lyophilization World. In addition to being a pharma-professional, he is also a regular speaker in webinar and a trainer on the topics like qualification and validations.
References:
ISPE Baseline Guide Vol 5: Commissioning & Qualification 2nd Edition.
EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary
Use Annex 15: Qualification and Validation