Control of Genotoxic and Elemental Impurities
ELEMENTAL IMPURITIES
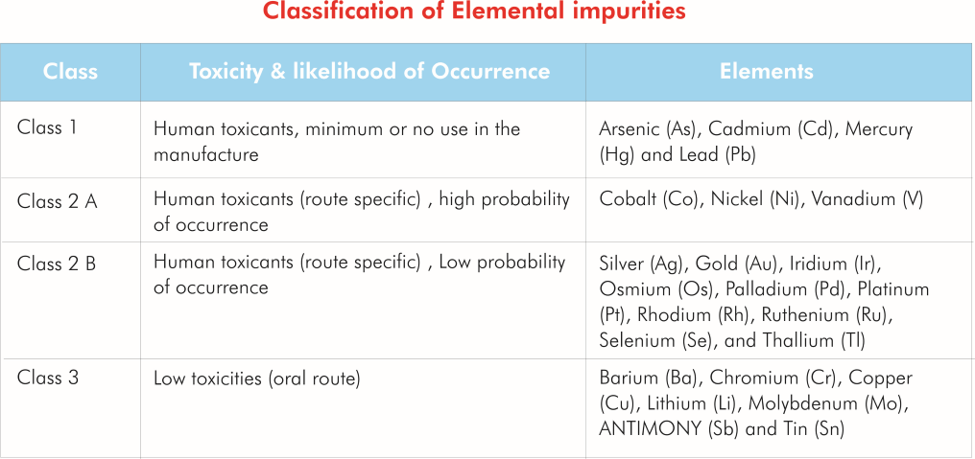
The presence of elemental impurities in drug products can potentially have adverse health effects.
Limits have been established for permissible amounts of elemental impurities in drug products.
Elemental impurities may include catalysts and environmental contaminants.
Elemental impurities may arise from natural occurrence in raw materials introduced inadvertently (for example from manufacturing equipment).
Element Toxic Effects
- Cd: Lung Cancer/ Stomach Irritation/Prostate
- Pb: Brain/ Kidney damage/Central Nervous system
- As: Skin Damage/Carcinogenic / Chromosomes
- Hg: Nervous Centre – Brain /Kidney/Fetuses
Existing Elemental Impurities Test: USP <231>
USP <231> is a test for “heavy metals”. Test indicates the total content of metal impurities using a colored sulfide precipitate.
It is in use since 1905. It is not element specific, but a visual (subjective) comparison test.
Standard solution color can change over time resulting in poor reproducibility. There are also issues with poor recovery when ash sample prep procedure is used.
Guidelines available on Inorganic Impurities and its estimation
- EMA ICH Q3D Guidelines (EMA/CHMP/ICH/353369/2013) −Effective from: 01/06/2016 (for new marketing authorisation applications) 01/12/2017 (for authorised medicinal products).
- USP General Chapters: USP Establishes January 1, 2018 as Implementation Date for Elemental Impurities.
- <232> Elemental Impurities—Limits in Drug Products
- <2232> Elemental Contaminants in Dietary Supplements
- Removal of <231> Heavy Metals