STANDARD OPERATING PROCEDURE, popularly known as SOP, is probably an essential document in the pharmaceutical industry and the most neglected documents compared with the other necessary documents. The Quality Management Systems documents such as Deviations, CAPAs, Change Controls, Effectiveness Checks, and Risk Assessments are considered prominent documents. Such prominence is usually not given to SOPs.
STANDARD OPERATING PROCEDURE, popularly known as SOP, is probably an essential document in the pharmaceutical industry and the most neglected documents compared with the other necessary documents. The Quality Management Systems documents such as Deviations, CAPAs, Change Controls, Effectiveness Checks, and Risk Assessments are considered prominent documents. Such prominence is usually not given to SOPs.
In this context will highlight SOP’s insight, its importance from a regulatory and organization point of view. Earlier, SOPs were usually not followed or written as per the standard; over a period, there had been a tremendous change in SOP and its design format. The perfect and effective written SOP is also a part of achieving the goals.
A Standard Operating Procedure (SOP) is a set of written instructions that documents employees follow a routine or repetitive activity in an organization. As industry experts, often fail to recognize that the mother of all evils is tendency to forget to follow SOP. In the pharmaceutical industry, the SOP activities begin from entering the premises, every internal action, and even how to exit the premises.
A typical Pharmaceutical Industry has an average of 400- 1300 SOPs. …. One must think that all procedures laid down, the industry must follow 100% correctly for smooth functioning. Unfortunately, it is not always true and the reason being the SOPs are not given the due prominence it deserves.
Breaking rules in social life creates some thrill to many individuals; many employees achieve the same hidden bliss by not following SOPs. Some old operators/supervisors even take pride that they don’t need any SOP to do their task and think they are perfect for executing the given task. Also, sometimes unintentional violation of SOP is also observed which may be due to multiple factors and human error. However, this is not good for the health of any organization. Rules, regulations, and SOPs help the operation run smoothly and as intended to achieve the target required from the equipment, process, or individuals.
WHY IS SOP REQUIRED?
SOP requires for almost all the activities processed in a pharmaceutical company. The general list of SOPs activities follows.
– To define a procedure for activities performed at the company site (manufacturing, cleaning, material handling, etc.)
– To operate machine / instrument / equipment/ utility
– To lay down policies (HR, entry-exit, health policies)
– To define systems and how to work with the system
– To handle documentation (change control, deviations, CAPA, batch records, logbooks)
– Any many more reasons.
WHY IS SOP IMPORTANT ? WHAT IS REGULATORY IMPACT?
Though SOPs state as a simple document, it is an essential procedure in the Pharma Industry. Regulators are very serious about observations; it is observed the employees do not follow site procedure
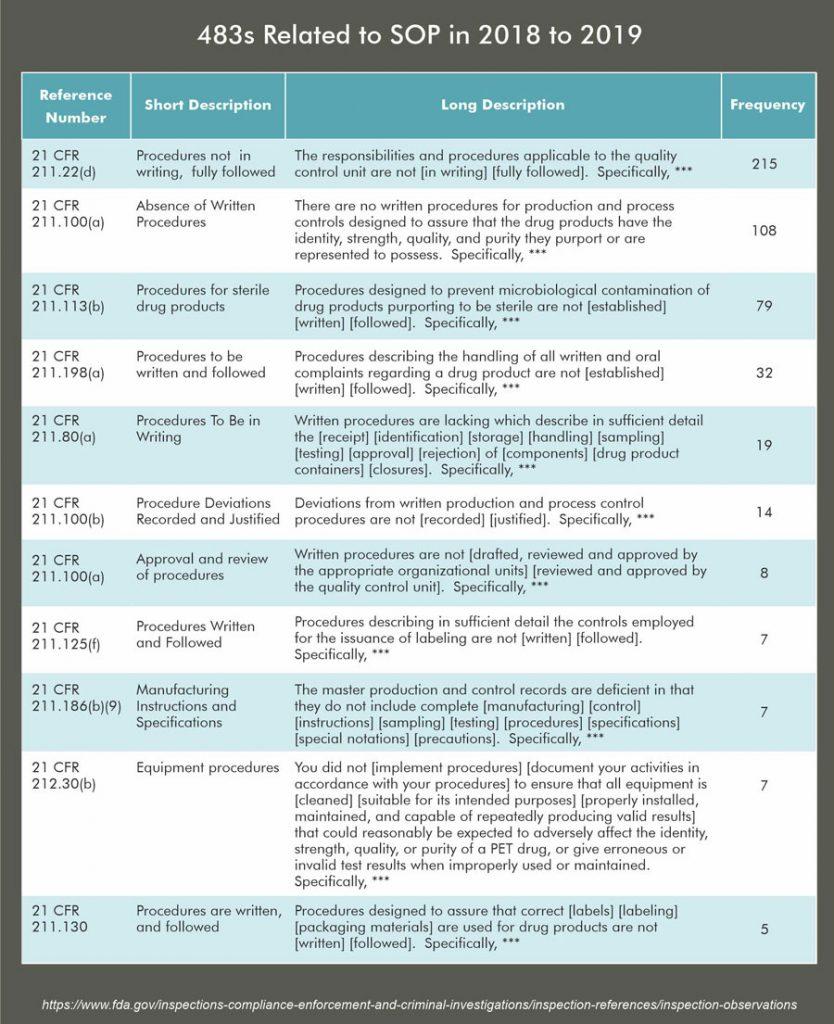
- One of the main reasons for Form 483 observations issued by FDA is that “SOPs are is not rigorously followed.”
- Ignoring or not following SOPS is like:
- a) Not doing things the way it design with compliance.
- b) Indicates that all activities at the site are fraudulent.
- c) Indicate potential data integrity issues
- d) Indicates the operation is not performed as per its validation and may result in process failure
WHAT GUIDANCE SAYS ABOUT SOPs AND IMPORTANCE OF SOP TRAINING?
21 CFR 211 SUBPART F PRODUCTION AND PROCESS CONTROL
Sec. 211.100 WRITTEN PROCEDURES, DEVIATIONS
(a) There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality and purity they purport or are represented to possess. Such procedure shall include all requirements in this subpart. These written procedures, including any changes, shall be drafted, reviewed, and approved by the appropriate organizational units and reviewed and approved by the quality control unit.
(b) Written production and process control procedure shall be followed in the execution of the various production and process control functions and shall be documented at the time of performance. Any deviation from the written procedure shall be recorded and justified.
Sec. 211.25 PERSONNEL QUALIFICATIONS
(a) Each person engaged in manufacture, processing, packing or holding of a drug product shall have education, training, and experience, or any combination thereof, to enable that person to perform assigned functions. Training shall be in particular operations that the employee performs and in current good manufacturing practice (including current good manufacturing practice regulations in the guidance and written procedure of 21 CFR 211 regulations) as they relate to employee’s functions.
Eudralex Annex-1
Good Manufacturing Practice Medicinal Products for Human and Veterinary Use:
It has emphasized on word ‘Procedure’ for all operations described in guidance and mentioned to have procedures defined and should be followed.
WHAT SHOULD BE CONTENT OF SOP?
The contents of SOP usually remain the same irrespective of formulation type, whether it is oral solids, liquids, injectables, external preparations, Etc.; however, if the processes change and so the writing of SOP content. Sometimes some minor variations can be seen between company to company – the usual content of SOPs.
# Purpose
# Scope
# Responsibilities
# References
# Steps to Follow in the sequence they actual process
# Precautions
# Notes
# Explanation of complex systems
# Parameters, limits, values
# Many more
What is the writing method of sop?
Perfect written SOP with steps in an exact sequence is easy to follow and avoids procedural deviations
SOP must be instructive and directive.
Use simple sentences and avoid complex English grammar or complex terminology (the reader may not be an expert in English, especially at operator level)
Always write SOPs in an Active voice. The supervisor should check the final written SOPs.
The detailed SOPS does not require any person to explain it.
Example: If SOP says ‘Select Program for garment load’ then it is incomplete instruction, but if it says ‘Go to recipe selection tab on HMI, select program No. 1 assigned for garment load cycle’ then it is detailed enough
Each step can be a maximum of three sentences. Not more than three sentences per step (not necessary, the long paragraph is boring to read, and sometimes readers can skip the critical information)
Example of short and long instruction
- Long Instruction
Take cleaned filling needles, pre-gassing needles, and post gassing needles. Take silicon tubing and clean by passing WFI for not less than 1min. Connect the silicon tubing to the needles and post gassing needle and again rinse the silicon tubing with needle and post gassing needle by passing WFI. Dry the parts with 0.2 µ filtered compressed air and cover the open ends with bio breathable paper. Keep filling needles, gassing needles and post gassing needle connected with silicon tubing in the SS tray.
- Short Instruction
- a) Take cleaned filling needles, pre-gassing needles and post-gassing needles.
- b) Take silicon tubing and clean by passing WFI for not less than 1min.
- c) Connect the silicon tubing to the needles and post gassing needle and again rinse the silicon tubing with needle and post gassing needle by passing WFI.
- c) Dry the parts with 0.2 µ filtered compressed air and cover the open ends with bio breathable paper.
- d) Keep filling needles, gassing needles, and post gassing needles connected with silicon tubing in the SS tray.
- The detailed SOP should be clear and easy to understand.
- Avoid direct statements of guidelines in SOP, convert the guidance words into actual practice to follow in the work area.
Example:
- Guidance for Industry September 2004 states that ‘Any manual or mechanical manipulation of the sterilized drug, components, containers, or closures prior to or during aseptic assembly poses the risk of contamination and thus necessitates careful control’ but it cannot use as instruction in SOP,
- SOP should state the sterilization methods and parameters, how to bring together and assemble sterilized items, what will be the aseptic practices while doing so, what care to be taken (slow movement, preventing lean over, operating through access gloves, etc.)
- Do not authorize decision-making to a doer; it may create confusion and can lead to deviation
For example, instructions like ‘select appropriate program’, ‘select suitable recipe’ are not giving explicit instruction about which program or recipe to be selected
HOW SOP BECOMES COMPLEX?
# QA usually people tend to seek CAPA for every root cause, even for rare errors. It also makes the SOP complex
# Every SOP writer is not an expert in MS Word. Sometimes to write a revised SOP quickly, to avoid significant format change, the writer finds the easiest way to prevent workload formatting. The instructions are placed, which requires the least format correction. In such a case, the written instruction does not match the sequence to the procedure, leading to repeated deviations resulting in making SOP more and more complex.
# In response to regulatory observations, inadequate SOP many times give rise to audit observations. In response, rather than correcting the issue from the root level, the writer uses the easy way or copy-pastes the procedure resulting in repeated errors, and further to fix the error SOP revision is quickly written and make the SOP more complex.
WHO SHOULD WRITE SOP?
– SOP writing requires the right amount of skill and experience on the topic. Points to consider while writing an SOP.
– SOPs should be reviewed by one or more individuals with appropriate training and experience of procedures covered in SOP. with the process.
– Do not assign SOP writing/revision tasks to an employee who is sitting idle.
– A person having through knowledge and experience on a given topic, an SME, should write SOP
– Involvement of SME in SOP writing / revision / review write perfect SOP, avoids unrealistic / unnecessary steps / instructions
– The SOPs should be written under supervision of by HOD’s of concerning departments and monitored by QA officer/ HOD QA, it can also must include the actual doer, shop floor person, the real executor, the operator, the down line supervisor who is daily involved in the activity as a team member of creating/revising SOP. It helps in avoiding SOP Vs Practice GAPs.
SOP REVISION
– Revision should be considered as critical while preparing a new SOP.
– Usually, SOPs are revised every two years or if there is a change in process or a revision required in response to CAPA arise from deviation, compliance with internal/external audit, and any process improvement identified by the execution team.
– There should be no restriction on SOP revision if anyone identified CAPA or gap against the practice. While revising the SOP against CAPA, also review the entire SOP for any other gaps that may be there and also correct the same during SOP revision. review the SOP for other gaps against the actual practice and do all changes simultaneously; it allows checking SOP Vs. Practice Gaps includes any update of guidelines and removes any flaws in the original version.
– SOP revision must follow the change control procedure to keep track of all modifications and identify the change in the new version. It helps to keep track records for the change in SOP when it comes to identifying, especially during and regulatory inspections.
– Revision history is also useful to keep track of changes made to comply with internal, external, or regulatory audits. These changes are a significant part of SOP until any change complies with the observation or regulations if identified. These changes should identify with a special marking, either making them inclined or bold letters so that it is easy to identify.
Common errors in SOP writing and how to avoid them
- Mismatched of instructed sequence: Keep SOP instructions in a sequence as per procedure. Example: Instruction to check utility pressures should come before starting the cycle, not after instructing to press the start button.
- Spelling mistakes: Can change the meaning of instruction, It creates a negative image about organization, review practice, and seriousness in the documentation, especially in front of an auditor
- Use appropriate words for the activity: Example: Remove, empty, unload are similar words; use an appropriate word which precisely defines the activity
- Avoid extra statements that have no direct relation to the action: Example: An information like “Equipment will generate an alarm in abnormal condition” is a general statement, and it doesn’t add value to SOP. Instead, include a separate section on alarm handling, a list of alarms, and for actions by each alarm
- Same instruction repeated multiple times: Avoid repeating the same instructions at multiple places. The terminology can differ when the same sentences repeated at numerous places. Also, while revising SOP, if same instruction in mentioned at multiple places then there is a high probability that revision in some places can be missed. can create confusion, and may need to miss to revise all instruction while SOP revision, can lead to deviation.
Example: In a SOP, instruction to wear mask may be included in multiple sections of SOP such as ‘general instructions at the start of SOP’, and then in ‘gowning procedure’ section and also in ‘process flow’ of gowning. When same instruction is placed at multiple places, there are possibilities that while revising these instruction, it can skip at one or more places.
- Avoid suspicious instructions like: ‘when felt necessary’, ‘if required’. The SOP must not have ifs and buts
- Provide the required value, limits, and parameters. Example: SOP instructs to check utility pressure before the start of cycle, pressure requirement for each utility like WFI, Steam, compressed air, nitrogen must be mentioned in SOP
- Any limitsation or parameters such as machines speed, product temperatures etc., must included in SOP with certified document.
- One can refer to another SOP for: the batch record, packing record. Provide specific references and avoid non-relevant references
- All explanations, instructions should be part of SOP: don’t include instructions to explain verbally, any verbal intimation.
- SOP should guide to QMS document to act upon: like, break down intimation note, in case of equipment failure
HOW REGULATORS LOOK AT SOPs?
Any pharma professional who had faced regulatory audits knows better to answers the questions, however, the main counter-question by auditor is ‘WHERE IT IS WRITTEN IN YOUR SOP?’.
From the regulator’s point of view, SOP prepared by an organization are essential documents that give them insight into the organization’s operation. If the auditor observes, too many deviations related to SOP violation OR SOP are not correctly written. It gives a message the organization is not serious about the operations, and management is paying less attention to quality of production to achieve intended quality.
This suspicion may make the auditor dig deeper, and if they found multiple deficient SOP or considerable deviations, it may result in a harsh audit outcome, which is not a healthy sign for any organization.
HOW COMPANY MANAGEMENT LOOKS AT SOPs?
From a management perspective, it is tough for higher management to digest the fact that the employees they hired to work for them have prepared deficient SOPs that are resulting in , SOPs causing a deficiency in regulatory observations. Management puts all efforts while hiring employees; the right people with the proper education and experience are part of the organization and capable of designing a compliant system and increasing productivity. When an audit observation emerges from deficient SOP or deficient designed systems, it creates a challenging situation for management to understand how it had happened? It may result in people losing their jobs and even their credibility in the market.
DO PEOPLE WRITE SOP OR JUST COPY-PASTE?
Writing a new SOPs is customary to apply previous experience or take references from available online data. Some contents from the equipment manual can prefer for writing SOPs. However, in doing so, sometimes, non-relevant information is incidentally becoming part of SOP and, if not correctly reviewed before implementing and can lead to deviation. If instructions are not in sequence, it may lead the to operators to find their own ways to perform the activity or operate the machine; as a result, it will be challenging to get the desired output offrom equipment or production, and one may never know the root cause for the same until detailed gap assessment between SOP and practice is done.

Standard Operating Procedure (SOP) – An unsung hero of pharmaceutical industry more…

SOP – Past / Present / Future : If we go back approx. 10-15 years, the writing of SOP’s was compact more…
Preview

Due to the rampant spread of COVID-19 in the country and the consequent travel restrictions more…

CPhi China more…

ACHEMA 2021 has postponed to April 4-8, 2022. This decision was taken after intensive discussions more…