The world has in the last one year, witnessed the biggest disruption unprecedented in history. The pandemic has made a phenomenal impact on the industrial world where the supply chains have been adversely affected. The Pharma Industry has been at the forefront as it was their role that came under the spotlight. We have seen that a lot of meaningful action that has been taken as things were moving at a snail speed before have suddenly picked up pace in the efficient implementation and execution. These quantum strides were very much required to make the industry provide solutions today for the issues of tomorrow…. could we have ever imagined the development of a vaccine in such a short period where a new vaccine can take anywhere upwards of 8 years. Globally we have today, over 100 vaccine candidates in different stages of development and a significant number of them in phase 2 and phase 3 trials.
The time has indeed come for the Pharma Industry to make a swift change by making use of the technology available whether it be the application of AI for the drug discovery processes or the use of robotics in drug substance handling and manufacturing to provide the much-needed safety to the humans and at the same time ensure that there is zero chance of contamination arising due to the human factor in the manufacturing process.
The first part of a series of articles on the Pharma industry here is to highlight the role of AI in changing the paper logs to digitization and monitoring on a real-time basis, in compliance with the regulatory authorities cannot be overemphasized but it needs to be understood as a basic requirement of pharma industry working going forward.
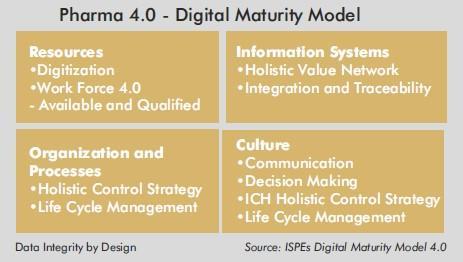
“Pharma 4.0 is a framework for adapting digital strategies to the unique contexts of pharmaceutical manufacturing. In practical terms, it means more connectivity, more productivity, simplified compliance, and the marshalling of production information to respond to problems as they emerge” – ISPE (International Society for Pharmaceutical Engineers)

The Pharma Industry is experiencing a major disruption in its various fronts and some of these areas are as under:
- Product differentiation
- Tremendous progress in Biotech, Genetics, Cell Therapy etc
- Treatment of rare and neglected diseases
- Geriatric population and their differing medicinal needs
- Mental health issues on the increase
- Customised medicines
- Pollution Challenges: Synthetics Vs Green Chemistry and enzymatic steps
- Ever-increasing stringent quality parameters and stricter FDA norms
- Supply Chain disruptions and need for cold chains to ensure product integrity
- Challenges like pandemic and virus mutation demanding continued vigil
- AI application in the medical domain to fast track drug discovery at reduced costs
The above is by no means is an exhaustive list of challenges but it spells a game-changer strategy that integrates quality into manufacturing with machines and technology as enablers with reduced or zero human intervention which will ensure cGMP compliance as laid down by the regulators.
We have noticed major transformation taking place in the growth of the pharma industry and all this has been possible due to the change agents, the stalwarts in the industry who could envision beyond the times and in their brainstorming sessions with the technology solution providers who together, worked upon to address and come with a workable solution now known as Pharma 4.0
The Pharma 3.0 was the process of Computerization and Connectivity and the journey from Pharma 3.0 to Pharma 4.0 included the following steps:
- Visibility- seeing what is happening
- Understanding why it is happening
- Predictive – what will happen?
- How an autonomous response can be achieved
Data Integrity By Design
We have been talking about Quality by Design – QBD for some years now and now is the opportune time to discuss Data Integrity by Design. In other words, the platform has changed from application integrity to data integrity. The systems today are moving to a design data and system architecture in which data integrity is intrinsic and part and parcel of the interacting collaborative platforms – interacting within various facets with the manufacturing space on the production floor seamlessly and transparently which facilitates online monitoring on a real-time basis
DATA INTEGRITY- MANUFACTURING SYSTEMS (MES) FOR PHARMA COMPANIES
The changing dynamics of drug substance supplies and the emergent need of medicines during the pandemic made some feel that the USFDA inspections became a little lenient. However, there is a growing need to go in for compliance at every stage to ensure that the right drug product reaches the patient despite all the compulsions and emergencies arising due to the sudden supply chain disruptions. There is a growing need for IT system solutions which minimize human error or rather does away with human intervention and captures digitally and in real-time basis every event in the raw material procurement, from raw material testing to dispensing, formulating of the drug substance, packing in all the stages namely primary, secondary and tertiary and thereafter stock tracking and ware-housing and right up to the last mile to ensure drug product integrity.
We find that the next big opportunity lies in the Pharma and Chemical industry. The Pharma industry on a worldwide basis has been a bit slow to implement such solutions which require allocation of funds and the decision has been delayed all this while. The situation as it stands, we have seen for ourselves how the revenues have been adversely impacted for the Pharma companies across the globe when there have been deviations and violations in cGMP compliances and companies have received import alerts and warning letters resulting in major revenue loss for substantial periods.
To address these aspects, we today find a large number of IT and data solutions companies focusing their attention on this opportunity for digitization and incorporating system solutions in the manufacturing and distribution processes to address the requirements of USFDA, EDQM, and other like regulatory authorities to ensure compliance with the regulations.
Some of the significant players offering the IT systems solutions are Schneider Electric, Lighthouse Systems, Emerson Electric Co., Pharma MES Berlin, General Electric Company, ABB Ltd, Siemens AG, Rockwell Automation, Werum IT Solutions GmbH, SAP SE, Dassault Systems, ATS Global, Atachi Systems & Honeywell.
Among the top global MES players listed above, Atachi systems is helping Indian pharma companies in succeeding in Pharma 4.0 journey with a tailored Next Generation MES solution (NGIMES).
We do find Indian companies also have sprung up in this space and one such name with a difference is Samvardhana Motherson Health Solutions (SMHS)– the healthtech arm of the auto-ancillary giant Motherson Group which has made an entry in the recent past and are providing configurable and customized IT solutions for healthcare like the elogbook, eBMR, centralized data systems, stock-tracking and warehouse management etc wherein they have competitive prices in comparison to some of the international names mentioned above. The emphasis is to replace paper and human intervention or else substantially reduce the incidence of human error creeping in the pharma manufacturing processes with digital technology platforms and further ensuring data integrity and compliances.
Performing Remote Audits During the Pandemic
During the COVID-19 pandemic, many of our planned on-site audits have given way to remote desktop audits. Each regulatory agency has its guidelines, but across the board, they all rely on the fundamental principle of a risk-based approach to compliance. As part of the audit preparation, it is important to ensure that all involved parties have suitable and compatible technological means in place, as tools and technology may vary considerably across organizations. A proper checklist may be prepared and shared in advance with minimum requirements, such as Internet upload and download speeds, screen sharing on a need basis to facilitate the audit process.
Therefore, we see that during the pandemic even the audit process can be facilitated by the use of technology and the same needs to have the following:
- Videoconferencing facility
- Web-based meeting systems with screen sharing capability
- Teleconferencing in addition to emails
- Secure file share systems
- ‘Read only' remote access to automated systems, such as electronic QM – quality management systems e.g to view (SOPs) - standard operating procedures, deviations, electronic batch records etc
AI, Robotics – applications in Pharmaceuticals and Bio-pharmaceuticals

The artificial intelligence pioneers developed machines that could think and reason like humans. The rapid growth in computing power and memory storage, an unprecedented wealth of data, big data, and the development of advanced algorithms led to substantial breakthroughs in Artificial Intelligence. AI applications today cover diverse fields like computer vision, voice recognition, language understanding, and digital pathology data analysis. Therefore, there lies an immense opportunity for AI in revolutionizing drug discovery by extracting hidden patterns, sequencing, and evidence from biomedical data. Today the pharmaceutical companies including some start-ups have used AI for drug discovery and development.
It is a well-known fact that the process of drug discovery or researching a New Chemical Entity (NCE) involves resources which have gone up from USD 800 Million to over USD 2 Billion and in addition, it requires many years of sifting various compounds and at times starting from approximately 10000 compounds and then coming down to one commercially viable efficacious drug substance for human use. Therefore, considering the substantial costs and resources, and a low success rate provides an efficient alternate in AI and its application in exploiting known drugs and thereby repurposing, etc.
‘Network medicine’- it is appropriate to make a mention of Network Medicine here which means science that combines principles and approaches from systems biology and network science in trying to understand the causes of human diseases and find and develop new treatments. While this becomes a bit technical for a layman reading this article but the application of AI to this aspect will have tremendous benefits to unravel the secrets and come to a desirable solution from a large number of permutation and combinations in the entire drug discovery and alternate application. For sure the knowledge of the interplay between drug targets and human diseases facilitated by AI algorithms can provide clues for possible drug repurposing in maybe in an economical way.
Pharma 4.0 Solutions
Plug & Produce
– Vertical integration through standardized interfaces between MES, automation, and control systems – which is a fundamental prerequisite for implementing various Pharma 4.0 solutions.
– Enterprise Manufacturing Intelligence (EMI)
– Translate production data into usable information for decision-making and improved processes.
Smart Devices
Connect and integrate mobile, and networked devices – making the right information available at the right time and place.
Pharma 4.0 – a way forward
In summary, we can say that Pharma 4.0 is a step in the direction of providing the industry with smart solutions, the changing of paper logs to the glass which means digitization which will become inevitable and necessary for enabling seamless data exchange across the entire pharmaceutical supply chain. This will mean the interconnectivity of people, product and processes will get integrated by the IIoT- the Industrial Internet of Things – The pharma and healthcare industry has been slow in getting into this space and now out of various factors of big data and need to have faster solutions at lower costs and lesser time would be the compulsions which will make the Pharma Industry get into IIoT and reap the benefits in a really big way. The analytics and the assistance in new drug discovery would require the Pharma industry to make much-needed investments in IIoT and for sure the Pharma industry stands to benefit many times over and therefore the companies which have done already or do it now will be the differentiating factor between the companies of the future and ‘also run’ ones. IIoT technologies will enable manufacturers to get a 360-degree view of their plant operations on a real-time basis with the ability to drill down to any level of detail at any stage of product development and manufacturing. Mind you, all this would be available at a tap of the screen or as they say on pressing of a button, of course, the buttons have now been replaced by flat screens and only a tap is required now.
The subsequent articles in this series on Pharma 4.0 would focus on the following aspects:
• Robotics in Pharmaceuticals – safety and contamination concerns
• Data Integrity and cCGM Compliance and Regulatory Audits
• Drug Repurposing – a solution that needs to be looked at
• Reshoring a Nation’s API and Pharmaceutical Production – ‘Reverse Swing’
• Pandemic and Supply Chain Disruption – Digital route to deliver Covid Vaccine in India.
https://www.thelancet.com/JOURNALS/LANDIG/ARTICLE/PIIS2589-7500(20)30192-8/FULLTEXT
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7474498/
https://web.uniroma1.it/stitch/node/5613
About author: Mr. Gurmeet Singh is Director Pharma Networks – a veteran for over 30 years in the Indian Pharmaceutical Sector. He worked with some of the best Pharma companies at the decision-making levels in companies like Ranbaxy, Wockhardt-Swiss and Ireland, Orchid Pharma, and Midas GmbH. In 2018 he worked as a Pharma Development Consultant with DNDi – Drugs for Neglected Diseases initiative – a Geneva-based R&D organization. Mr. Singh is currently associated with Samvardhana Motherson Health Solutions Limited as Business Development Manager. Mr. Singh is a Bachelor of Law (LL.B), a Post Graduate in Management Studies, and a Post Graduate Diploma in Intellectual Property Rights Law. His coordinates are [email protected] and [email protected]

Standard Operating Procedure (SOP) – An unsung hero of pharmaceutical industry more…

SOP – Past / Present / Future : If we go back approx. 10-15 years, the writing of SOP’s was compact more…
Preview

Due to the rampant spread of COVID-19 in the country and the consequent travel restrictions more…

CPhi China more…

ACHEMA 2021 has postponed to April 4-8, 2022. This decision was taken after intensive discussions more…