Enhancing Pharmaceutical manufacturing with AI and ML

 Rameshwar Verma is a part of the leadership team of an esteemed pharmaceutical company as an Executive – Packing (Serialization Specialist) with 8+ years of experience.
Rameshwar Verma is a part of the leadership team of an esteemed pharmaceutical company as an Executive – Packing (Serialization Specialist) with 8+ years of experience.
He has good knowledge about serialization system, pharmaceutical packing, industry 4.0, QMS, electronic records and compliance. He is the author of the books: “Basic concept of track and trace system for pharmaceutical industry” and “Pharmaceutical track and trace system: A guide to optimizing efficiency and compliance”.
Artificial intelligence (AI) and machine learning (ML) are reshaping pharmaceutical manufacturing processes. These technologies, from intelligent automation to personalized therapies, are crucial in ensuring the pharmaceutical industry’s efficiency, compliance, and patient safety.
Artificial intelligence (AI) and machine learning (ML) are no longer futuristic concepts; they are now part of how we live and work. The U.S. Food and Drug Administration uses the term AI to describe a branch of computer science, statistics, and engineering that uses algorithms or models to perform tasks and exhibit behaviors such as learning, making decisions, and making predictions. ML is a subset of AI that uses data and algorithms, without being explicitly programmed, to imitate how humans learn.
What is artificial intelligence (AI)?: AI is a machine’s ability to display human-like capabilities such as reasoning, learning, planning and creativity.
Why is AI important? Artificial intelligence is seen as central to society’s digital transformation and has become a priority for regulatory bodies.
Types of AI:
• Software: virtual assistants, image analysis software, search engines, speech and face recognition systems.
• “Embodied” AI: robots, autonomous cars, drones, Internet of Things
It focuses on using a risk-centered strategy to assess and manage AI/ML in promoting advancements and safeguarding public health.
There are challenges associated with AI/ML in pharmaceutical manufacturer such as:
• Ethical and security considerations like improper data sharing or cybersecurity risks.
• Concerns arise when using algorithms that lack transparency, or algorithms that have hidden internal operations that users or other interested parties cannot see.
• Lead to increase in errors or reinforce existing biases in the data.
• AI algorithms require large amounts of data to train and optimize their models. This data includes information on raw materials, manufacturing conditions, and quality control measures in drug manufacturing. Gathering and organizing such data can be time-consuming and challenging.
• Limitation is the lack of regulatory guidance for AI in drug manufacturing. AI introduces new considerations regarding validation, quality assurance, and regulatory compliance. Regulatory agencies need to establish clear guidelines to ensure the safety and efficacy of AI-assisted drug manufacturing processes.
• Connecting manufacturing/Packaging equipment to a network can be challenging in preserving product quality and ensuring the privacy and security of the data generated by the equipment. This is especially important in the pharmaceutical manufacturing industry.
• Incorporating AI into current manufacturing systems brings technical hurdles. Concerns like compatibility, data integration, and system interoperability must be resolved for successful AI integration in drug manufacturing.
• The level of human involvement is crucial and will vary based on the application of technologies.
Despite, these challenges and limitations, AI has the potential to revolutionize drug manufacturing. By addressing these challenges and leveraging AI’s full potential, the pharmaceutical industry can enhance productivity, improve product quality, and accelerate the development of life-saving medications.
Artificial Intelligence (e.g. NLP) & Machine Learning can be applied to pharmaceutical manufacturing processes as given below:
• Various types of quality management system (QMS) records, such as deviations, CAPAs, OOS, OOT, change controls, and complaints, are maintained to enable the monitoring and trend analysis of these records. These records not only help identify problem areas but also facilitate predictive analytics.
• In the past, analyzing and choosing QMS data to support tasks like manufacturing or complaint investigations was typically done using methods like 5 Whys, Brainstorming, Ishikawa, Rule in Rule Out, etc. However, these processes were time-consuming and often only gathered a small amount of important data or even collected irrelevant data.
• Packaging operations applications could predict potential failures, support/trigger adaptive actions, and avoid unexpected downtime and stoppages.
• AI/ML techniques analyze images or videos of glass, medications, packaging, and other items to determine if they meet quality standards. This can involve identifying defects, measuring dimensions, or creating maps showing where issues are located.
• AI algorithms can continuously monitor manufacturing processes, detecting deviations from standard operating procedures and alerting operators to take corrective actions promptly.
• ML models can analyze sensor data and predict equipment failures, allowing manufacturers to schedule maintenance activities proactively and prevent production downtime.
• AI algorithms can identify abnormal patterns or behaviors in data, helping manufacturers detect potential quality issues early and take preventive measures.
• ML techniques can analyze large datasets to identify trends, correlations, and potential risks, enabling manufacturers to optimize processes and make informed decisions.
• AI and ML can automate repetitive tasks, improve inventory management, and optimize resource allocation, increasing productivity and reducing waste.
• AI-powered systems can monitor product quality in real-time, detect anomalies, and ensure compliance with regulatory standards, ensuring the delivery of safe and effective medications to patients.
• By automating repetitive tasks, companies can reduce labor costs and minimize the risk of human error, resulting in significant cost savings.
Integration of AI with Industry 4.0 Technologies
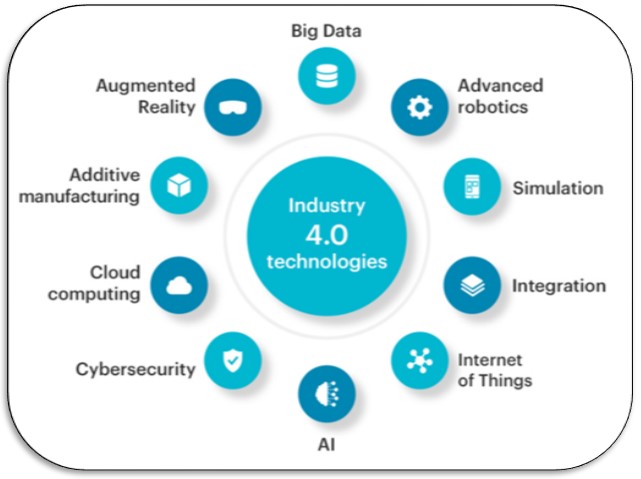 Integrating AI and Industry 4.0 technologies brings new possibilities to healthcare, transforming procedures and facilitating progress in predictive maintenance, real-time data analysis, and autonomous robotics.
Integrating AI and Industry 4.0 technologies brings new possibilities to healthcare, transforming procedures and facilitating progress in predictive maintenance, real-time data analysis, and autonomous robotics.
Industry 4.0 is built on nine technology pillars. These innovations bridge the physical and digital worlds and make intelligent and autonomous systems possible. Businesses and supply chains already use some of these advanced technologies, but the full potential of Industry 4.0 comes to life when they’re used together. These technologies include:
1. Big Data and AI analytics
2. Vertical and horizontal integration
3. Cloud computing
4. Augmented reality
5. Industrial Internet of Things
6. Additive manufacturing (also known as 3D printing)
7. Autonomous robots
8. Simulation or digital twins
9. Cybersecurity
• Internet of Things: The Internet of Things (IoT) is a network of connected objects and devices equipped with sensors (and other technologies) that allow them to transmit and receive data – to and from other things and systems. Today IoT is used broadly in industrial settings (IIoT) and is synonymous with Industry 4.0.
• Big Data Analytics: Big Data is the ocean of information we swim in daily—vast zettabytes of data flowing from our computers, mobile devices, and machine sensors.
• The true value of Big Data is measured by the degree to which you can analyze and understand it. Artificial intelligence (AI), machine learning, and modern database technologies allow for Big Data visualization and analysis to deliver actionable insights – in real time.
• Big Data analytics help companies put their data to work–to realize new opportunities and build business models. As Geoffrey Moore, author and management analyst, aptly stated, “Without Big Data analytics, companies are blind and deaf, wandering out onto the Web like deer on a freeway.”
“Data is the lifeblood of AI. An AI system needs to learn from data in order to be able to fulfill its function.”
-Brandon Purcell, analyst, Forrester Research
Let’s now focus on how AI is integrated with Industry 4.0 technologies. This integration has great potential for the industry by merging AI and ML with smart manufacturing and cyber-physical systems. To help you understand this integration better as per below table: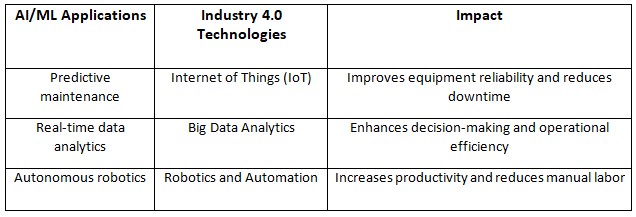
AI/ML in pharmaceutical manufacturing can work alongside “other advanced manufacturing technologies (eg, process analytical technology, continuous manufacturing)”, helping to enable the implementation of Industry 4.0.
Conclusion: the integration of AI and ML in pharmaceutical manufacturing processes has the potential to revolutionize the industry by enhancing efficiency, improving product quality, and accelerating the development of life-saving medications. However, some challenges and limitations, such as ethical and security considerations, lack of regulatory guidance, and technical hurdles, need to be addressed. By overcoming these challenges and leveraging the full potential of AI, the pharmaceutical industry can benefit from increased productivity, reduced waste, and cost savings. Additionally, integrating AI with Industry 4.0 technologies further enhances processes through predictive maintenance, real-time data analytics, and autonomous robotics.
Reference:
- Artificial Intelligence and Machine Learning (AI/ML) for Drug Development | FDA
- Artificial Intelligence Discussion Paper (fda.gov)
- How Artificial Intelligence is Revolutionizing the Pharmaceutical Industry – RandomTrees – Blog
- Industry 4.0 technologies – BPI – The destination for everything process related (businessprocessincubator.com)
- What is artificial intelligence and how is it used? | Topics | European Parliament (europa.eu)
- Industry 4.0 Solutions from SAP | IIoT & Smart Manufacturing Software
- Big Data: The Next Frontier for Business | SAP
Articles
By Rameshwar Verma
An Interview with
President Maurizio Marchesini
Embracing Digital Innovations and Sustainable Practices Amidst Regulatory Pressures
An Interview with
Avinash Kumar Talwar







