
The hygienic design of equipment and utilities is of great importance in pharmaceuticals and healthcare to avoid contamination risks (both microbial and chemical). Good design, the aims and expectations of cleaning validation can be achieved.
In the previous two issue, we discussed Surface Composition (Part 1) and vessels and Pipes (Part 2), this article is about Pumps, Seals, and Valves, The third article highlights the hygienic design considerations of pumps, seals, and valves. It also consolidates the key takeaways from the previous articles to discuss the important aspects of quality by-design and its regulatory implications
The proper design of equipment and utilities plays a vital role in the pharmaceutical and healthcare industries in order to prevent contamination risks, both microbial and chemical. By implementing effective design principles, the objectives and expectations of cleaning validation can be met. Failing to achieve good hygienic design not only poses a risk to the product but also has economic consequences, such as increased operating costs due to factors like higher energy consumption and additional plant downtimes (1).
In the case of pumps, their cleanliness largely depends on their design, as an optimal hygienic design aims to minimize crevices and eliminate stagnant areas where bacteria and chemicals can accumulate. If pumps cannot be cleaned-in-place, the ease of disassembly for manual cleaning and the nature of material surfaces become crucial factors.
Hygienic requirements should be addressed during the design and development stages, starting with the selection of suitable pump manufacturers who can demonstrate design compliance with industry standards. The hygienic design of equipment needs to be subsequently assessed by the user to show that the desired cleanability of the equipment can be achieved.
Similarly, valves need to be designed with hygiene in mind. It is imperative that valves work efficiently to control fluids and that their housing sealing is consistently reproducibly cleanable. It should be “gapfree”. It is important that product residues do not accumulate in the gaps due to the low flow velocity in this area and hence efficient cleaning becomes challenging.
As for seals, their design should prevent soil and microbial build-up by being completely free of gaps under all operating conditions. Even the smallest gaps and crevices can harbor microorganisms or chemical residues, potentially leading to product contamination (2).
This article examines the essential hygienic design features of pumps, valves, and seals, and synthesizes the content covered in the three-part article series.
Pumps
It’s crucial that pumps have self-draining capabilities. When self-drain is not possible, due to design or operational constraints, Pumps can be moved into a position to be drained. Pumps should also have open crevice-free hygienic joints for optimal cleaning.
Mono pumps should be avoided because of their poor design and potential for product impeller breakup. Tri-lobe pumps, in contrast, are a well-liked hygienic design. The lobes should be positioned carefully to prevent abrasion. It is important to be aware of the connections and to avoid products becoming trapped. Additionally suggested are peristaltic pumps. This kind of pump operates by gently pumping a tube. The peristaltic pump has advantages when used with shear-sensitive products and specialized equipment, such as a tube, is needed (3).
To calculate the flow rate through a pump (single acting plunger or piston pumps), the following calculation can be used:
Q = DP2 × L × n × N × π / 924
Where:
Q = Flow Rate in gallons per minute
DP² = Plunger/Piston Diameter in inches
L = Stroke Length in inches
n = Number of Plungers/Pistons
N = Speed of pump in revolutions per minute
π = Pi = 3.14159
Hence:
Flow Rate [GPM] = (Plunger Diameter [in])2 × Stoke
Length [in] × Number of Plungers × Pump Speed [RPM] × 3.14159 / 924
As an example:
• Plunger Diameter = 3.5 inches
• Stroke Length = 6 inches
• Number of Plungers = Triplex = 3
• Pump Speed = 100 RPM
And:
(3.5 [in])2 × 6 [in] × 3 × 100 [RPM] × 3.14159 / 924 =74.97 GPM
Seals
The design of sealings is one of the major aspects of hygienic design. Improperly selected seals represent areas where contaminants can reside. The sealing design needs to avoid the accumulation of soil and microbes and therefore has to be ‘gap free’ under all operation conditions. Even very small gaps and crevices can harbor a large population of microorganisms and hence this can be a source of product contamination. Areas around shaft seals should not contain deep annular crevices and the seals must be cleanable. A hygienic seal design can
protect process øuid from environmental contamination. The use of springs in the product contact area should be avoided.
Valves
Effective flow control and sampling systems require essential valves in closed systems, complex processes, and complex technologies commonly found in the pharmaceutical industry. The valves play a crucial role in regulating, directing, and controlling fluid flow by opening, closing, or partially obstructing it within a system. This functionality operates in conjunction with pressure differentials, as fluids naturally flow from higher to lower pressure areas.

Image 2: Ball valves (Heather Smith / Alloy Valve Stockist, licensed under a Creative Commons Attribution 3.0 License).
Various types of valves can be utilized for processing purposes, ranging from unhygienic options like ball valves to hygienically designed butterfly valves. A ball valve is a quarter-turn valve that employs a hollow, perforated ball to manage flow. It opens when the ball’s hole aligns with the flow and closes when pivoted 90 degrees by the valve handle. On the other hand, a butterfly valve serves to isolate or regulate fluid flow. Its closing mechanism consists of a rotating disk, commonly referred to as the “butterfly,” mounted on a rod. When fully closed, the disc completely blocks the passageway, while a quarter turn in the fully open position allows unrestricted fluid passage.
Image 3: Butterfly valve (Alloy Valve Stockist, licensed under a Creative Commons Attribution 3.0 License).
Valves that are considered unhygienic are those with housing sealings that cannot be reproducibly cleaned, allowing residue buildup in the gaps, due to low flow velocity thus efficient cleaning becomes impossible. Residues not only present a risk for contamination of subsequent product batches but they are also a safe harbor for microorganisms to grow and protect them during sterilization or disinfection. These residues increase the risk of microbial contamination of subsequent product batches produced on the same process line. In contrast, hygienic valves act as both the static seal (shell seal) and a dynamic seal (weir shutoff). (4)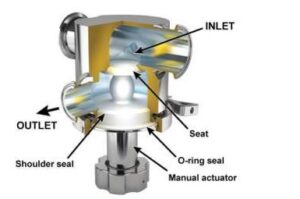
Image 4: Zero dead leg valve (Aspeco, licensed under a Creative Commons Attribution 3.0 License).
Diaphragm valves (or membrane valves) The valve body with two or more ports, an elastomeric diaphragm, and a “weir or saddle” or seat upon which the diaphragm closes the valve. The valve body may be constructed from plastic, metal, wood, or other materials depending on the intended use. There is a possibility of a tear in the diaphragm valve.
These tears can cause contamination from fluids that get entrapped in the diaphragm. Diaphragm tears can be hard to detect since the pressure boundary of the diaphragm is not breached, the in-line instrumentation does not detect a system problem.
There are some hygienic diagram valves available; these have valve bodies that are made of stainless steel and wetted surfaces. Such valves are electropolished or machined to a fine finish, as designed to be cleaned-in-place.
A further good design feature with valves is the use of zero deadleg valves which are used to minimize deadlegs in critical areas of the piping system. In less critical areas, it was permissible for valves to be connected with tees or bends.
Mixers
Mixing in pharmaceutical manufacturing serves various purposes, such as combining raw ingredients, preparing emulsions, reducing particle size, conducting chemical reactions, manipulating rheology, dissolving components, and facilitating heat transfer. Consequently, many pharmaceutical product lines utilize different types of mixers to process raw ingredients, handle intermediates, and prepare the final product.
Image 5: Powder mixer in a pharmaceutical facility (Adinhath, licensed under a Creative Commons Attribution 3.0 License).
The size of the mixer depends on the required throughput. It is crucial for mixers to be easily cleaned to prevent the contamination of chemicals between batches (5). To prevent batch-to-batch contamination, CIP and Steam-in-Place (SIP) capabilities are typically incorporated, along with features like air-purged seals and custom-designed discharge valves.
Instruments
The instrumentation used to assess the process also needs to be hygienic. Key criteria include:
•The materials used should be resistant to corrosion and easy to clean. One option is to use 316L stainless steel with a fine surface finish. (aiming for a Ra surface reading of 0.51 µm or better). It should be noted that the processes involved in making some active pharmaceutical (API) ingredients are highly corrosive and may demand more exotic alloys, such as those with higher nickel levels.
•Any crevices and corners where bacteria and other contaminants can accumulate should be eliminated. Welds need to be smoothed both inside and out, and interior corners should have a radius. Conventional pipe threads should never be used.
•Pockets and dead legs in piping should be avoided. Liquids should not have any areas where they can be trapped or retain residues. Piping should be pitched to ensure self-draining, and surfaces should be as smooth and flush as possible.
Other considerations for hygienic design
In addition to the main areas of hygienic design mentioned earlier, it is also important to consider the following:
•Hygienic design of heat exchangers, this includes double-plated exchange plates to prevent leakage, ensuring they are fully drainable, and incorporating a failsafe mechanism for the coolant/heating side.
•Have a close look at the gasket, seal areas, and product traps, to ensure they are effective in maintaining hygiene.
•Ensuing that filter housings are drainable.
Engineers, quality personnel, microbiologists, and others must grasp the fundamentals of hygienic design in order to provide effective oversight. The microbiologists must be included early in plant, and equipment acquisitions and updates (such as the change control process). This is crucial in case of any modifications during installation. Such actions necessitate risk assessment, which focuses on topics such as: How will risks be controlled? How will the system be sanitized after the task is completed? How many samples are needed to evaluate the impact of the work?
To attain these objectives, microbiologists must collaborate successfully with engineering, maintenance, and production units to keep sanitary practices high and at the forefront of operations.
Maintaining hygienic security and good quality design considerations
Once systems are correctly constructed and operational, it is critical to maintain hygienic oversight, inspect systems on a regular basis, and review the available data, whether physical, chemical, or in relation to bioburden. Effective planned preventative maintenance should be in place, and any type of replacement should be made at suitable time intervals. Preventative maintenance, which entails a program and strategy for maintaining equipment, can help facilities save money and reduce downtime.
Adopting a failure-oriented mentality is not a smart practice because the failure outcome can provide considerable microbiological danger.
Good hygiene design principles are outlined with examples discussed in this article. Effective hygienic systems and equipment in units is a regulatory expectation, such as the U.S. Code of Federal Regulations, notably Subpart C – Building and Facilities Section 211.42 Design and Construction Features, which states:
•Adequate size, construction, and position must allow for easy cleaning, maintenance, and proper operation.
•Plan adequate space for the orderly positioning of equipment and materials to avoid mix-ups and contamination.
•Design the adequate flow of materials and persons to prevent contamination.
A sound approach to hygienic design is also in keeping with ICH Q8, which defines the design space on the basis that quality cannot be tested into the product but has to be built in through good design (6). ICH Q8 needs to be considered alongside the principles of quality risk management, as presented in the sister guideline document ICH Q9 (7). Quality risk management is most effective when used (8):
•Prospectively.
•On the basis of straightforward, objective models created with the greatest science and engineering information, knowledge, and tools available.
•Led by an objective, interdisciplinary, and experienced team of subject matter specialists.
Together, these two standards represent ‘quality by design’. Quality by Design is defined as “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” in the ICH Q8 guideline. This philosophy includes:
•Product quality and performance can be assured by designing efficient manufacturing processes.
•Product and process specifications are based on a scientific understanding of how process factors affect product performance.
•Risk-based regulatory techniques for scientific comprehension and process control connected to product quality and performance.
•Related regulatory regulations and actions are revised to reflect current scientific understanding.
•Quality assurance as a continuous process.
While a comprehensive contamination control strategy, attention also needs to be given to the control of the environment (as with cleanrooms), control of personnel and traffic movement, and process design. These concerns can be presented as three aspects (9):
•Process: unit operations, grouping of unit operations into logical operational units; appropriate process equipment, components, and instruments; automated process control systems; and input and in-process materials and products.
•Facilities: structures, environmental systems, layouts, operational flows, logistical support, utility systems, and other building control systems such as the building management system.
•Infrastructure: Practises, procedures, people (training, discipline, and qualification), maintenance systems, and automated procedural control systems that regulate the facility and process elements (such as manufacturing execution systems, electronic batch records, and so on).
This chapter primarily focuses on process design, which is a crucial aspect among others. It’s important to note that other aspects, especially in understanding how equipment meets hygienic design requirements. It plays a significant role in understanding how poor practices can inadvertently re-contaminate.
The pharmaceutical sector has drawn inspiration from the food industry to improve hygienic design practices. However, the pharma industry has historically lagged behind the food industry in terms of innovations related to equipment specifications, design, and cleaning (10).
Cleaning is a complex process influenced by various factors, including the type of dirt to be removed, cleaning duration, temperature of the cleaning agent, and the hydrodynamic force of the moving liquid (10). This article, along with the other two in this series, discusses essential aspects of equipment hygiene and cleanliness, providing measurements and calculations to assess the hygienic status effectively.
Summary
As mentioned in this series of articles, improperly specified and designed processing equipment can have a negative impact on the microbiological and chemical safety of the product, and may even lead to physical contamination. Therefore,
it is crucial that all equipment that comes into contact with the product is designed with hygiene in mind. The application of hygienic design involves reviewing all relevant project stages. This can be facilitated by project management tools that aim to minimize risks and identify the most cost-effective designs to maintain the desired hygiene levels.
This article was the third and final in a series focusing on hygienic design concepts. Drawing on some of the points raised in the previous articles, a quality-driven approach to hygienic design should consider:
| Material | Surfaces | Construction |
| Corrosion resistant | No holes | Dead leg free |
| Chemically resistant | No gaps | Corners > 90° |
| Nontoxic | No crevices | Radii > 3 mm (ideally, > 6 mm) |
| Non contaminating | No folds | No sharp edges |
| Nonabrasive | Cleanable | No metal to metal connections (bolds/nuts) |
| Non absorbing | Capable of being disinfected | Self-draining |
| No influence on product | Capable of being sterilized, if required | Low number of gaskets |
| Temperature resistant | Roughness Ra ≤ 0.8 µm | Stable gap-free sealing construction |
The hygienic design of pharmaceutical processing is crucial for ensuring the microbiological safety and quality of products. It helps prevent high microbial counts, toxins, and chemical residues in products at various stages of manufacturing. By prioritizing hygienic requirements and using proper equipment that is operated and maintained correctly, a facility can produce safe and high-quality products.


ABOUT THE AUTHOR
Tim Sandle is the author of the book Digital Transformation and Regulatory Considerations for Biopharmaceutical and Healthcare Manufacturers, Volume 1: Digital Technologies for Automation and Process Improvement, available via the PDA Bookstore: https://www.pda.org/bookstore/product- detail/5897-digital-transformation-volume-1




