Driving digitalization of products and supply chains
 Antares Vision Group is driving digitalization of products and supply chains by leading traceability, inspection, and integrated data management. AV Group helps companies and institutions to achieve safety, quality, efficiency, and sustainability, enabling Trustparency®.
Antares Vision Group is driving digitalization of products and supply chains by leading traceability, inspection, and integrated data management. AV Group helps companies and institutions to achieve safety, quality, efficiency, and sustainability, enabling Trustparency®.
It guarantees the quality and integrity of products, from production to market, by creating a unique digital identity for each saleable item. AV Group enables traceability from raw materials to the end user while ensuring compliance with regulatory authorities.
In Life Science industry Antares Vision Group providescomprehensive solutions, including hardware and software, to meet global regulatory compliance requirements across the supply chain, from manufacturing to logistics and verification operations, distribution and retail.
Tracking and Tracing products at any point in the value chain is beneficial for any industry. Track & Trace solutions for pharmaceutical improve product safety by reducing the possibilities of counterfeiting and guarantee the reliability of the supply chain. By implementing lifetime traceability with Track & Trace solutions, it is possible to follow and monitor products throughout their life cycle.
A complete Track & Trace process, made of serialization and aggregation, represents the best possible control, as it could tell the whole history of any given product – from the manufacturer to the point of dispense, giving lifetime traceability across the supply chain.
 The unique identifier becomes the “digital passport” that not only enhances the protection of brands but also enables production line monitoring and minimize recalled products and guaranteeing several benefits:
The unique identifier becomes the “digital passport” that not only enhances the protection of brands but also enables production line monitoring and minimize recalled products and guaranteeing several benefits:
• Compliance with worldwide regulations and directives
• Get information from line to optimize production and distribution efficiency
• Facilitate recall operations
• Control product diversion
• Guarantee quality and originality of the product
• Ensure transparency of supply chain
• Get information about end users and shelf life
Serialization and Aggregation Solutions
Antares Vision Serialization and Aggregation Equipments are designed to manage operations in production plants and warehouses:
• Compact, standalone serialization modules optionally combining advanced packaging functions (eg. Checkweigher, Tamper Evident Labelling) for quick deployment and line space saving.
• Print & Check Serialization kit integrated on 3rd party OEM equipment.
• Stand-alone automatic unit that performs all serialization functions, including marking, recording and verifying all serialization data, ensuring data consistency and security under all conditions.
• Semi-automatic or Manual Standalone Aggregation Modules for end-of-line packaging operations.
• Automatic Aggregation kits designed for installation on bundling machines, case packers and palletizing stations.
Track & Trace Software Solutions
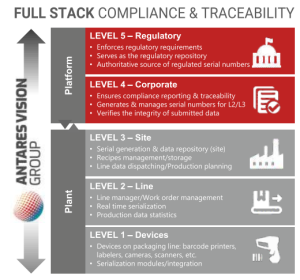
Based on its unparalleled experience in installing track & trace on manufacturing lines, Antares Vision Group has developed ATS – Antares Tracking System – the fully integrated software platform to manage all serialization, aggregation, data storage and exchange activities.
ATS has proven its functionality and efficiency across thousands of serialization lines deployed worldwide. This includes current serialization and aggregation regulations in Turkey, South Korea, Argentina, China, EU-FMD, DSCSA and other international pharmaceutical product safety regulations.
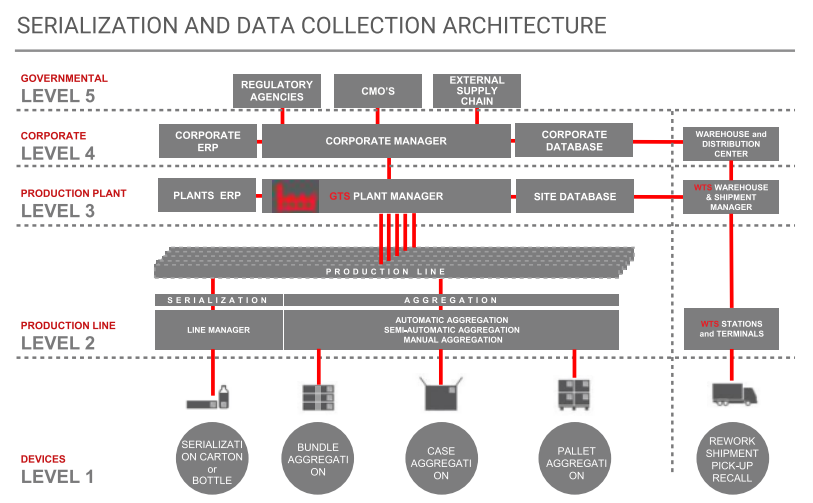
ATS can manage all tracking data at line, plant or corporate level. Antares Tracking System coordinates line-level serialization of individual pharmaceutical packs, cases up to pallet. At plant and corporate level, ATS can interface with the internal ERP to manage order handling and exchange of serialization data with external supply chains and regulatory agencies
Key Benefits:
• Worldwide Regulation Compliance Expertise: Most widely installed solution
• Overall Scalability: Corporate, Production, and Warehouse Environment can be integrated with existing solutions
• Total Flexibility: New Devices, Procedures and Business Rules can be easily changed or added, and Independently Validated
• User-friendly Interface





