
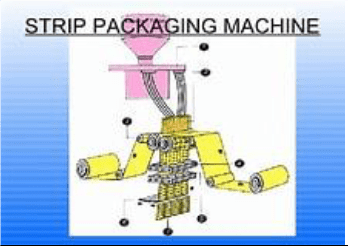
I, Masih Jaigirdar, by education am a Pharmacist and by profession a Pharmaceutical Scientist started my professional career in the early 70s. In my boyhood in mid 50s when I used to go to the pharmacy or the medical college hospital dispensary to get the prescribed medication, they used to dispense the tablets either in a paper package or amber colour glass bottle. However, over the years it changed into different mode of dispensing, especially when the capsule medication was made available in the sixties; then the packaging was usually done by the pharma industry making strip packaging putting individual tablet or capsule into a pocket created between polymer laminated aluminum foil going through two heated rollers in a machine (e.g. Hasia or others).

Medicine packer bottles pharmaceutical grade
Medicine packer bottles pharmaceutical grade
However, with my retirement, before I go into relaxation and hibernation mode, thought to bring as food for thought on certain pharmaceutical cum medical issues that I have come across over my career of about 50 years. I still remember after my graduation of Pharmacy joined the then E.R.Squibb & Sons, in the early 70s in their pharmaceutical plant in Bangladesh. At that time we have used Hasia Strip Packaging Machine for tablets and capsules packaging. We have packaged their Theragran-M multivitamin tablets and others with this strip packaging machine.
Packaging of Solid Dosage (Individual unit) overseas
In the early 70s when I started my career in Squibb, BD, it was “Aluminum-foil Strip packaging”, putting a single unit tablet/capsule within a pocket created between two polymer laminated aluminum foil passing through two heated knurled rollers with circular/elliptical cavities/sockets drawing the tablet/capsule from the hopper through a chute by a strip packaging machine with regulated temperature melting the polymer at the knurling area of the roller to seal the strip for the each individual unit of the dosage. A cutting knife was employed to cut the strip based on required number of units of dosage. An in-process test was done for the sealing check by performing a leak-test in a beaker/device with
water, and putting one of such sealed strip with tablets/capsules and exerting vacuum. If there would be some leakage in the one of the pocket or many pockets of the strip (mostly at the beginning of the operation) air bubbles will come. Then after thoroughly wiping this strip undergone for the leakage test with towel or dry tissue, the strip was opened carefully to find which pocket tablet/capsule had actual wetness. This procedure was employed for both initial setting of the machine as well as an in-process test for the packaging process.
In mid-80s when I went to Sweden for training at the then ASTRA at Sodertalje, Sweden, to take the position of Head of the Process Technique for their newly built pharmaceutical plant in the M.E; it was pre-pocket formed “Blister” packaging. Now it was initial or simultaneous pockets created in a hard see-through polymer either colourless or with yellowish or reddish colour required to protect against light. Individual unit tablet/capsule is put inside the pre-formed pocket and then closing and sealing mostly with a thin aluminum foil laminated with polymer, by either a single unit strips packaging or continuous striping packaging machine. Initial testing and in-process testing were somewhat similar to that of aluminum foil strip packaging.
The user or the patient would open only one unit dosage from any of this system of packaging for intake. Hence, both of these above systems/procedures of packaging will not only provide protection against product stability towards its shelf-life but also any pilferage, contamination and/or physical manipulation for any ill-motive.
Packaging of Solid Dosage (Individual unit) in the US
However, unlike overseas, in the US it is still use of bottles for the solid dosage packaging. These are packaged/supplied in bottles of High Density Poly Ethylene (HDPE) of counts for 30s, 90s or 500s up to 5000s unit with pilfer proof caps either child resistance (CRC) or non-CRC, aluminum foil induction liner, with or without dunnage and with or without desiccant supplies for use by the patient taking medicine or as commercial package for re-packaging in the pharmacy. This practice of pharmaceutical packing and supply for the US market is century old and due to the prejudice and un-scientific mind of the population. To me this packaging system has many disadvantages for the following reasons:
Product Stability: Pharmaceutical companies for its solid dosage drug products would put into a container of various sizes in the following configurations depending on number of units to fill-in and its stability requirement: a) single-walled, double-walled, with dunnage (polyester) or no-dunnage, no or canisters of different sizes, desiccant (for moisture protection), odour absorption (activated charcoal) or both depending on the requirements.

Pharma HDPE CRC bottle (US)

Prescription pharmacy CRC vials-Amber (US)
I myself as a product development formulator at various phase of product development, used to put in bare petri-dish and intended/planned product containers for the above stated sizes and configurations and requirements at the following stability conditions i) Controlled Room Temperature (CRT) using shelving cabinet ii) Stress stability condition 50°C / 2-3 Weeks iii) Accelerated Stability Condition (40°C / 75% RH) for 1, 2 and 3 months; and Long Term Stability Condition and iv) Accelerated Light Condition.
Generally during the product development stage for the packaging stability testing, the following critical process parameters are maintained:
The commercial supplier warehouse and/or the pharmacy will receive in such packaged conditions ensured by the pharmaceutical manufacturer for their drug products.
However, these drug products would again undergo the following stages of handling by the pharmacy or the patient during the dispensing or actual usage (intake) steps that may have impact on the stability of the product.
Packaging of Solid Dosage (Individual unit) in the US
The pharmacy for 90 days supplies to the patient, generally will dispense these 90 counts of the dosage units tablet/capsule from a bulk container of at least more than 100 to 5000 count bottles and take into a tray for counting and filling into their own clear amber colour bottle and close with a cap and label it with all required information.
The concern here is; there are two types of compromising:
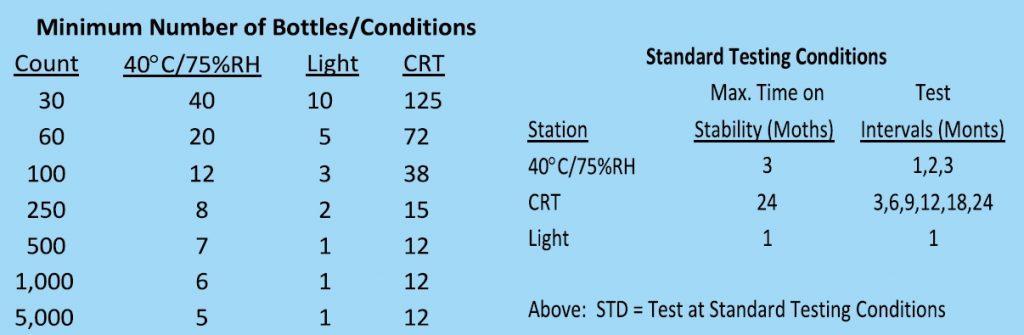
P.S: With this studied the acceptability stability was determined and accordingly the required packaging
configuration and CCPs are established and finalized.
- How the stability of the remaining dosage units in the bulk container is maintained once it is being opened. Thus firm given/maintained required stability condition is lost. If there were desiccant it will ultimately absorb the room humidity of the pharmacy storage.
- When the pharmacist/technician is spreading the dosage units on a dispensing tray they are exposed not only to the room temperature, humidity conditions but also other in-borne particulate of the room air supply. Thus contaminating and/or compromising product stability condition provided by the drug product manufacturer.
An Example of Packaging System Used in the Pharmaceutical Industry (Generic)
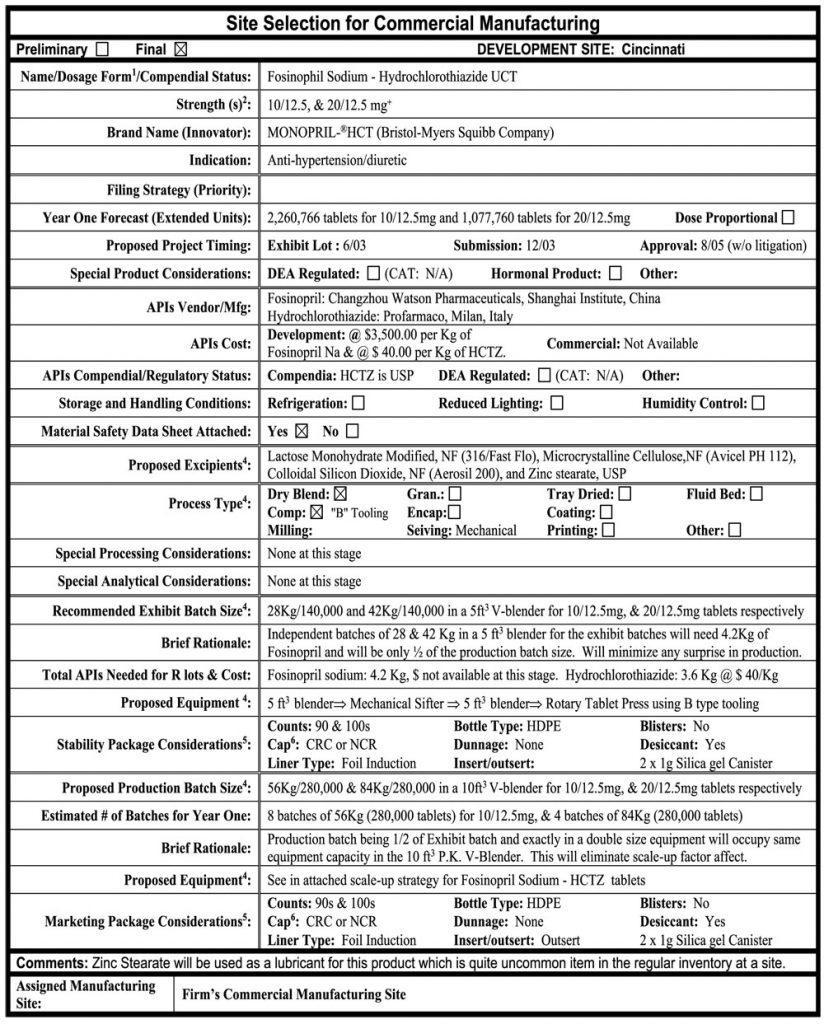
Point of concern and suggestion:
What is the CGMP regulatory guideline and the current cGMP practice for the pharmacy environmental control of monitoring and recording all required CPPs i.e., temperature, humidity and personnel hygiene for dispensing and secondary packaging. Otherwise all those precautions required during the packaging of pharmaceutical unit dosage at the industry would be lost and the quality of the DP would be lost.
At actual patient use stage:
Generally at home, be it for 30s or 90s, the patient or his/her care taker will place this above stated dispensed drug product tablet/capsule in a medicine cabinet most likely within the vicinity of the bath-room where there is possibility of high-humidity and in winter temperature conditions and being opened at least for 30 to 90 times based on supplied format. Thus further exposing the medication at such stability compromised conditions.
Comments and Suggestions:
Here, my concern is without performing actual studies on these factual exposures of the drug product what would be their final stability, i.e., assay, impurity and/or pharmacological effectiveness?
My humble suggestion is: the academia should think about it and make some scientific studies to determine, come up and publish what is needed to be done? Otherwise due to this years old practice and stubborn attitude in the US population of having the medication (solid dosage) packaged, supplied, and dispensed will ultimately compromise it quality and efficacy as opposed to the overseas packaging system used for the solid dosage medication in blister package.
For such studies the college of pharmacy pharmaceutics with the collaboration of the agency (FDA) can do by simulating the conditions such as:
I. For the pharmacy condition: Simulating the opening of the bulk container where it is supplied in an induction sealed high density polyethylene (HDPE) bottle in bulks (1000s); for the worst scenario for 30 units DP (Tablet or capsule) dosage X number of such numbers of opening and closing simulating in that prevailing environmental condition of the pharmacy dispensing area (temperature and humidity). Do all analytics including, assay, dissolution and impurity to find if the last dosage has maintained these quality integrity.
 II. For the at actual patient use stage: Simulating the opening of the dispensed container where it is supplied in an Prescription Pharmacy CRC Vials-Amber in 30s or 90s for one month or three months supplies; for the worst scenario for 90 units DP (Tablet or capsule) dosage X number of such numbers of opening and closing simulating in that prevailing environmental condition at home in a medicine cabinet most likely within the vicinity of the bath-room where there is possibility of high-humidity and in winter temperature conditions. Do all analytics including, assay, dissolution and impurity to find if the last dosage has maintained these quality integrity.
II. For the at actual patient use stage: Simulating the opening of the dispensed container where it is supplied in an Prescription Pharmacy CRC Vials-Amber in 30s or 90s for one month or three months supplies; for the worst scenario for 90 units DP (Tablet or capsule) dosage X number of such numbers of opening and closing simulating in that prevailing environmental condition at home in a medicine cabinet most likely within the vicinity of the bath-room where there is possibility of high-humidity and in winter temperature conditions. Do all analytics including, assay, dissolution and impurity to find if the last dosage has maintained these quality integrity.
I just wanted to share my own experience and related concern, of going through the pharmaceutical packaging and dispensing evolution which I have come across in my life and over these 50 years being in the US and the practice of DP dispensing and taking of medicine by ultimate user, the patient.







