A present investigation aimed to evaluate the impact of commonly used different binder-diluent ratios on the tablet’s mechanical strength to propose a binder-diluent classification scale. The addition of low and high fragmenting diluent in the predominantly plastic binder leads to Class III (high reduction) and Class IV (extremely high reduction) changes in the resulting tablet’s mechanical strength. The impact of predominantly brittle diluent on the tablet mechanical strength of plastic binder was less the low and high fragmenting materials. Interestingly, a synergistic or positive impact on the tablet’s mechanical strength was found after the addition of high fragmenting diluent into low fragmenting diluent. A change in the tablet mechanical strength of plastic binder is a direct function of the adopted diluent-binder ratio and applied compression pressure. A high compression pressure could compensate for the negative impact of fragmenting and brittle diluents on the mechanical strength of plastic binder tablets. Certainly, a proposed scale could serve as a ‘guiding torch’ for various formulators while designing preclinical or early-phase formulations.
Direct compression and dry granulation are the first and second choices of tablet manufacturing due to their simplicity as compared to the cumbersome wet granulation process [1]. Direct compression has a limited number of inter-processing steps. It can therefore pose fewer challenges for continuous tablet manufacturing as per the ICHQ8 guideline [2]. One of the downsides of direct compression is their inability to process bulkier and poor-flowing powders. This can be handled with a second-choice dry granulation process [1].
 Dry granulation is also a “method-of-choice” for various high-load bulkier drug formulations specifically small molecule oncology new chemical entities (NCEs). Such NCEs belong to BCS Class-II to Class-IV leading to their high dose. As per our experience, oncology tablet formulation could have API from 30% to 80%. This implies the 30 to 80 % dominance of API on the final tablet formulation properties with limited room for the excipients amount. This warrants a smart selection of multi-purpose excipients while developing the formulations to avoid Phase-II to late development tablet manufacturing issues such as capping, lamination, picking, and sticking (CLPS).
Dry granulation is also a “method-of-choice” for various high-load bulkier drug formulations specifically small molecule oncology new chemical entities (NCEs). Such NCEs belong to BCS Class-II to Class-IV leading to their high dose. As per our experience, oncology tablet formulation could have API from 30% to 80%. This implies the 30 to 80 % dominance of API on the final tablet formulation properties with limited room for the excipients amount. This warrants a smart selection of multi-purpose excipients while developing the formulations to avoid Phase-II to late development tablet manufacturing issues such as capping, lamination, picking, and sticking (CLPS).
CLPS issues are difficult to capture in the early development stage. These issues become more prominent in the late-stage development phase, thanks to the large batch size as compared to early phases. Such late-development issues become very challenging because of the boxed formulation. These troubled formulations are difficult to change owing to their involved time and money expenditure. Certainly, it is important to understand vital components of formulations such as multi-purpose excipients even in the pre-clinical stage of formulation development.
Tablet formulations are composed of two or more excipients serving the purpose of binder and/or diluent. They provide desired strength to maintain the tablet’s integrity. Common binders and diluents used in the formulations are microcrystalline cellulose, lactose, dibasic calcium phosphate (Emcompress®), and mannitol. Formulation scientist employs different ratios of these materials to achieve the desired mechanical strength of the tablet for robust tableting. These binders are predominantly plastic and brittle in nature. Hence, it is important to evaluate the impact of a diluent-binder replacement on the compact strength of a directly compressible tablet.
This before-hand knowledge could be extrapolated to the dry granulation process if necessary. The present investigation aimed to evaluate the impact of different binder-diluent ratios on the tablet mechanical strength of resulting tablets. Finally, a binder-diluent classification scale has been proposed. This scale could serve as a ‘guiding torch’ for various formulators while designing their direct compression or dry granulation formulations.
Materials and Methods
Different predominantly deforming various common binder-diluents such as microcrystalline cellulose (plastic) [3], α-lactose monohydrate (low fragmenting) [3], Emcompress® (high fragmenting) [3], and mannitol (brittle) [4] were chosen.
These materials were sieved from Sieve # 40 and then blended in 25:75, 50:50, and 75:25 ratios in a lab-scale V-shape blender.
A single component and bi-component diluent-binder 10 mm flat-faced tablets of these materials were manufactured by compressing powder at 100 MPa, 200 MPa, and 300 MPa using Natoli RD-10A single station press.
Results and Discussion
An impact of mixing binder and diluent in the different proportions is given in Figure 1. As expected, predominantly plastically deforming microcrystalline cellulose produced stronger tablets in the absence of other materials.
The tablet mechanical strength of microcrystalline cellulose tablets decreased with the addition of fragmenting (low and high) and brittle materials such as α-lactose monohydrates (Figure 1A), Emcompress® (Figure 1B), and mannitol (Figure 1C). It was interesting to observe that the addition of these materials in the microcrystalline cellulose was leading to a 16% to 85% reduction in the tablet mechanical strength of microcrystalline cellulose. These changes were the direct function of material type and their amount.
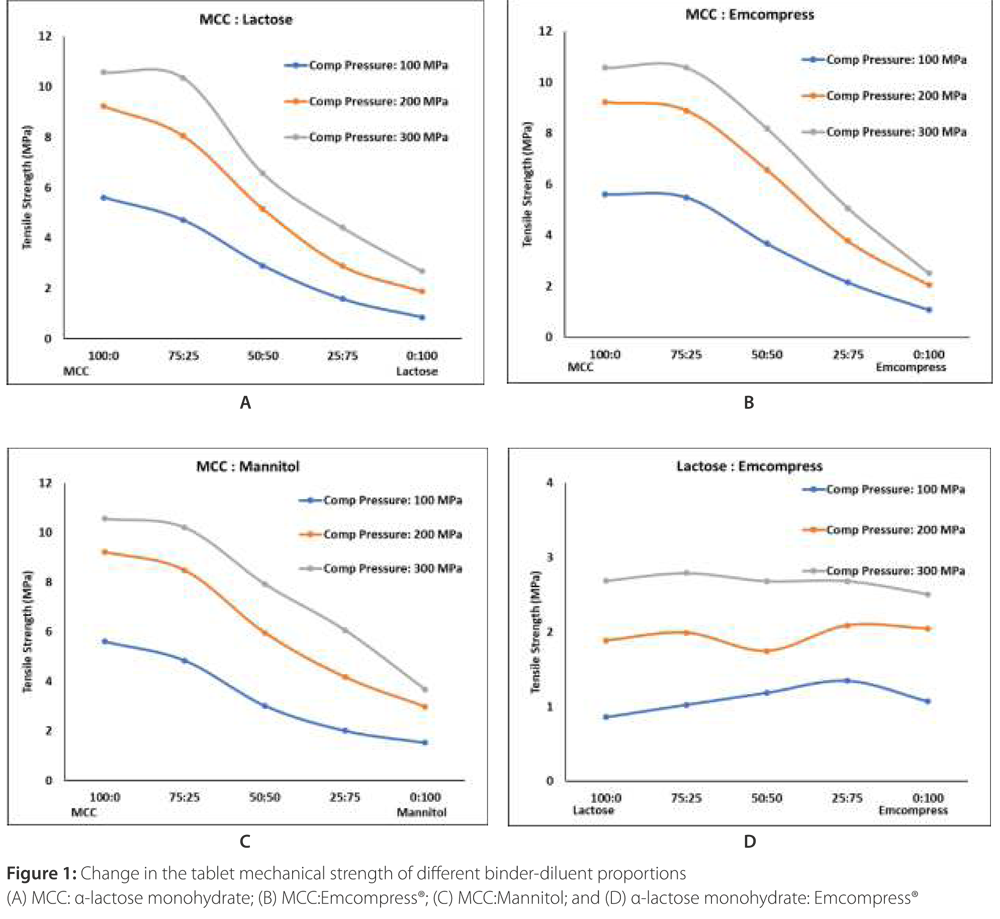 Addition of low fragmenting α-lactose monohydrates (Figure 1A) and high fragmenting Emcompress® (Figure 1B) reduced microcrystalline cellulose tablet mechanical strength from 2 to 85%, and 2 to 81 %, respectively. This could be attributed to particle shielding of the predominantly plastic microcrystalline cellulose due to the fragmentation of these materials in the early compression cycle. Such particle shielding could impair the plastic deformation of microcrystalline cellulose particles. This translates as a weakening of the particle bonding [5], and subsequent tablet mechanical strength reduction of even very good binders like microcrystalline cellulose by 72%.
Addition of low fragmenting α-lactose monohydrates (Figure 1A) and high fragmenting Emcompress® (Figure 1B) reduced microcrystalline cellulose tablet mechanical strength from 2 to 85%, and 2 to 81 %, respectively. This could be attributed to particle shielding of the predominantly plastic microcrystalline cellulose due to the fragmentation of these materials in the early compression cycle. Such particle shielding could impair the plastic deformation of microcrystalline cellulose particles. This translates as a weakening of the particle bonding [5], and subsequent tablet mechanical strength reduction of even very good binders like microcrystalline cellulose by 72%.
Tablet weakening of plastic-deforming materials in presence of fragmenting and brittle materials could be compensated by increasing the compression force. However, this reduction could be compensated between 88 to 213% and is mainly dictated by the amount of fragmenting material. A relatively least impact of high fragmenting Emcompress® on microcrystalline cellulose tablet mechanical strength could be attributed to the increased number of bonding due to extensive fragmentation, which is some amount compensated for the reduced bonding due to impaired plastic deformation of microcrystalline cellulose [6]. An addition of brittle mannitol reduced microcrystalline cellulose tablet mechanical strength by 3 to 73% (Figure 1C). A less negative impact of mannitol as compared to α-lactose monohydrates could be imparted to limited fragmentation and the particle shielding effect of brittle mannitol [4, 5].
 However, a synergistic effect of the addition of high fragmenting Emcompress® into low fragmenting α-lactose monohydrate tablet mechanical strength was observed (Figure 1D) specifically at 100 MPa compression pressure. The addition of Emcompress® into α-lactose monohydrate increased its tablet mechanical strength between 19% to 56% at 100 MPa compression pressure. This could be attributed to an increased number of bonds due to the improving fragmentation of the resulting blend [6].
However, a synergistic effect of the addition of high fragmenting Emcompress® into low fragmenting α-lactose monohydrate tablet mechanical strength was observed (Figure 1D) specifically at 100 MPa compression pressure. The addition of Emcompress® into α-lactose monohydrate increased its tablet mechanical strength between 19% to 56% at 100 MPa compression pressure. This could be attributed to an increased number of bonds due to the improving fragmentation of the resulting blend [6].
However, a limited increase (0.37% to 11%) in the tablet mechanical strength of α-lactose monohydrate with the addition of Emcompress® was observed when the compression pressure was 200 MPa and 300 MPa. It could be attributed to the increased fragmentation of α-lactose monohydrate at high compression pressure. This synergistic effect was further accelerated by 100 to 213% with an increase in the compression force.
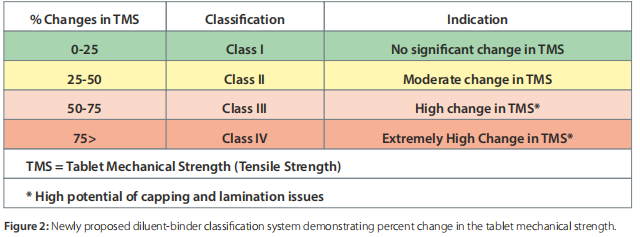
The addition of plastic, brittle, and fragmenting (low and high) materials showed a reduction or increase in the tablet’s mechanical strength in comparison to the single-component systems. Thus, a binder-diluent scale (Figure 2) is proposed based on this preliminary data. A change in the tablet’s mechanical strength was classified into four classes. Class I, II, III, and IV changes were defined as the reduction in tablet mechanical strength by 0-25%, 25-50%, 50-75%, and 75-100%, respectively.
A binder-diluent mixture exhibiting Class I and II changes could lead to insignificant to moderate changes in the tablet’s mechanical strength. These changes could be less sensitive to CLPS issues. On the other hand, binder-diluent mixtures displaying Class III and IV changes might experience a high to extremely high reduction in tablet mechanical strength. These mixtures will highly have the chance of CLPS issues.
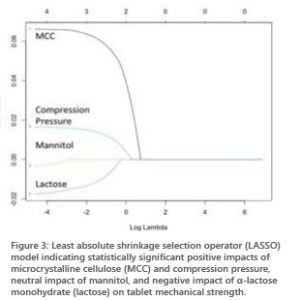 Current findings were further validated statistically with the least absolute shrinkage and selection operator (LASSO) model (Figure 3). This model exhibited a neutral to positive coefficient (correlation) compression pressure, and microcrystalline cellulose with tablet mechanical strength, while mannitol and lactose exhibited a negative coefficient (correlation) with tablet mechanical strength.
Current findings were further validated statistically with the least absolute shrinkage and selection operator (LASSO) model (Figure 3). This model exhibited a neutral to positive coefficient (correlation) compression pressure, and microcrystalline cellulose with tablet mechanical strength, while mannitol and lactose exhibited a negative coefficient (correlation) with tablet mechanical strength.
Conclusions/Key Findings
 The addition of brittle, low-, and high-fragmenting diluents in the predominantly plastic binders such as microcrystalline cellulose exhibited Class III and Class IV change in its tablet mechanical strength as per the newly proposed classification system. Such changes could pose significant CLPS issues in the late development stage. These changes are a direct function of the binder-diluent ratio, which could be compensated to some extent with the increase in compression pressure.
The addition of brittle, low-, and high-fragmenting diluents in the predominantly plastic binders such as microcrystalline cellulose exhibited Class III and Class IV change in its tablet mechanical strength as per the newly proposed classification system. Such changes could pose significant CLPS issues in the late development stage. These changes are a direct function of the binder-diluent ratio, which could be compensated to some extent with the increase in compression pressure.
Interestingly, the addition of a high fragmenting diluent with a low fragmenting diluent exhibited a synergistic or positive impact on the tablet mechanical strength of the former ones. However, a binder-diluent mixture exhibiting a Class I and a Class II change in the tablet’s mechanical strength might produce tablets with the desired integrity.
Certainly, these preliminary findings might serve as a ‘guiding torch’ while selecting the combination of various commonly used binders and diluents in the direct compression or dry granulation formulation development to the various formulators across the globe.
References:
- Patel, S., A.M. Kaushal, and A.K. Bansal, Compression Physics in the Formulation Development of Tablets. 2006. 23(1): p. 1-66.
- https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development Accessed on 02/09/2023.
- Haware, R.V., I. Tho, and A. Bauer-Brandl, Application of multivariate methods to compression behavior evaluation of directly compressible materials. European Journal of Pharmaceutics and Biopharmaceutics, 2009. 72(1): p. 148-155.
- Tarlier, N., et al., Deformation behavior of crystallized mannitol during compression using a rotary tablet press simulator. Int J Pharm, 2018. 547(1-2): p. 142-149.
- Haware, R.V., A. Bauer-Brandl, and I. Tho, Comparative evaluation of the powder and compression properties of various grades and brands of microcrystalline cellulose by multivariate methods. Pharmaceutical Development and Technology, 2010. 15(4): p. 394-404.
- Alderborn, Particle Dimensions, in: G. Alderborn, C. Nystroem (Eds.), Pharmaceutical Powder Compaction Technology, vol. 71, MarcelDekker, Inc., New York, 1996, pp. 245-282.