Good Powder Flow Is Everything!
By Fred Rowley
On a tablet press or encapsulator, good powder flow is everything! It is a basic yet paramount need. You cannot produce quality products or solve problems until you establish good powder flow. In tablet or capsule manufacturing, powders must flow like a liquid. Essentially the powder must take the form of a liquid and flow into the die unassisted, says Fred Rowley, an internationally recognized solid dosage expert and a pioneer in solid dosage training. Pharma Machines & Technology met with him at the seminar on “Why blame the Excipients?” organized by the International Pharmaceutical Excipients Council of India (IPEC India). Further to the personal discussion and in response to our queries, he writes for us in this exclusive on solid dosage manufacturing.
The objective is to place the same amount of perfectly blended powder in each hole. We call this hole the “die”. And the dies are the same shape as the punches; meaning that dies can have very unusual shapes. In order to accomplish this task the powder must flow along a horizontal surface we call the “die table”.
Therefore, powder must flow like a liquid on a tablet press or encapsulator to flow evenly and undisturbed from the powder hopper through the feeder and onwards into the die. Many problems, such as potency problems and soft tablets are the result of poor powder flow.
 Fred A. Rowley, President, Solid Dosage Training, Inc. is an internationally recognized solid dosage expert and a pioneer in solid dosage training. He has been Director, Manufacturing Technical Support, for Watson Laboratories; Plant Manager, Tablets and Capsules, Weider Nutrition International; Vice President, Operations, Arnet Pharmaceuticals; OROS Operations Manager, Alza Corporation; and Bulk Solids Manager, Syntex FP, Puerto Rico.
Fred A. Rowley, President, Solid Dosage Training, Inc. is an internationally recognized solid dosage expert and a pioneer in solid dosage training. He has been Director, Manufacturing Technical Support, for Watson Laboratories; Plant Manager, Tablets and Capsules, Weider Nutrition International; Vice President, Operations, Arnet Pharmaceuticals; OROS Operations Manager, Alza Corporation; and Bulk Solids Manager, Syntex FP, Puerto Rico.
He received his B.S. Biochemistry degree from the University of Santo Tomas, Manila, Asia. He was nominated for a doctorate degree, honoris causa, in 2006. Mr. Rowley has written many technical articles in the fields of tablet granulation, compressing, coating and printing and currently serves on the editorial advisory boards of both Pharmaceutical Manufacturing and Tablets and Capsules magazines. He has been retained as a solid dosage manufacturing expert by various law firms and pharmaceutical companies in 22 different countries. He authored 32 peer reviewed articles and lectures at both the university and professional industry levels in the United States, Asia, Europe and India in all areas of solid dosage manufacturing.
Poor powder flow in tablet compression
The natural shape and size of the particles of individual powders present in a tablet formulation may be quite different. And there might be as many as eight different powders in a formula. Further, the powders may be functional but there is no guarantee that they necessarily flow. And if you then combine multiple powders having this basic problem you also increase the difficulty of moving them uniformly into that small die.
Powders must flow like a liquid!
 The best analogy we can use to demonstrate this relationship is to liken the powder blend to a liquid. Liquids are relatively easy to fill into a container even with only basic controls like a flow control. A great comparison is to observe several bottles of a soft drink or bottled water: there is little variation between the contents. Now imagine that the soft drink is the powder blend and the plastic bottle is the die on a tablet press, and you begin to appreciate the difficulty. Now imagine that tablet press filling 3,000 dies per minute and you begin to understand.
The best analogy we can use to demonstrate this relationship is to liken the powder blend to a liquid. Liquids are relatively easy to fill into a container even with only basic controls like a flow control. A great comparison is to observe several bottles of a soft drink or bottled water: there is little variation between the contents. Now imagine that the soft drink is the powder blend and the plastic bottle is the die on a tablet press, and you begin to appreciate the difficulty. Now imagine that tablet press filling 3,000 dies per minute and you begin to understand.
Lack of knowledge
Generic pharmaceutical companies suffer from a lack of knowledge in the manufacturing department. When developing a formula, generic pharma companies tend to copy the established formula of the innovator company. Why try to reinvent the wheel? So qualitatively the formulas are usually similar. The method used to improve the flow of that formula may be very different resulting in varying degrees of success in their improvement of the overall powder flow. For example, trying to compress the Metformin 500mg tablet of 5 different generic companies can range from easy to very difficult based wholly or in part on how the powder blend flows.
University versus Industry
Students in research departments often manufacture small formulas of less than 1-2 kg. At this level, flow problems may or may not be present. As this initial formula is scaled up to the commercial level, to as much as 4,000 kg, flow problems may begin to appear. At this point the formula has usually already been submitted to the regulatory authorities and may not be readily changed. As a result the manufacturing department is now “stuck” with this formula for the life of the product.
Science versus Skill
Both science and skill have their place in successful tablet manufacturing. The science relies on the formula the R & D department develops while the skill is usually assumed to mean the adjustment of critical tablet press machine settings during tablet manufacturing. The science is present in the form of machine set point ranges stated on the approved batch record while the skill is the selection of the exact set points for this particular drug within the range.
have their place in successful tablet manufacturing. The science relies on the formula the R & D department develops while the skill is usually assumed to mean the adjustment of critical tablet press machine settings during tablet manufacturing. The science is present in the form of machine set point ranges stated on the approved batch record while the skill is the selection of the exact set points for this particular drug within the range.
Injectables versus Tablets
People not normally exposed to pharmaceutical manufacturing believe that injectables are sterile compounds that are extremely difficult to manufacture whereas tablets are a simple powder compressed until a tablet is formed. But the exact opposite is usually true: tablets are complex blends of powders blended to an exact potency and compressed into tablets at very high speeds.
Troubleshooting in Solid Dosage
Troubleshooting in solid dosage is almost never quick, easy, and free. Tablet manufacturing is burdened with the fact that a single defect may have as many as 17 different root causes. Reviewing each one of these root causes takes time and money. Further, even with an established formula a slight change of a single raw material may result in an otherwise satisfactory formula turning into a highly problematic powder blend to compress successfully. Tablet press operators will often remark that although the formula has not changed, the materials have not changed and the tablet press has not changed, different batches compress differently.
dosage is almost never quick, easy, and free. Tablet manufacturing is burdened with the fact that a single defect may have as many as 17 different root causes. Reviewing each one of these root causes takes time and money. Further, even with an established formula a slight change of a single raw material may result in an otherwise satisfactory formula turning into a highly problematic powder blend to compress successfully. Tablet press operators will often remark that although the formula has not changed, the materials have not changed and the tablet press has not changed, different batches compress differently.
It’s a constant issue!
Flow issues remain constant on the production floor. Different conditions such as temperature and humidity shifts as well as slightly different machine set points may turn an otherwise well performing powder blend into a problem. For example, storage conditions are notorious for altering powder flow. Even several bins of powder stored at different distances from a room air conditioning vent may create different powder flows when finally compressed on a tablet press. And the time difference between the actual compressing of the individual bins might even be an additional factor.
Why do we continue to have flow issues on the production floor?
But only with some products; not all.
- After multiple formula adjustments in R&D
- After scale up and “validation” batches
It remains a constant issue to solve
- And in some cases it is never solved
So, therefore…
- The concept of understanding and preparing particle size/shape be the entire answer!
- Yet excipient manufacturers are pressed to provide an answer as if it is their fault.
The heart of the matter: A powder blend must flow, compress and eject!
- Some formulas are almost all drug (API): So the API usually both flows and compresses.
- Other formulas have very little API: So the powder blend then reflects the attributes of the excipients.
- But almost all of the excipients in the formula are not there to increase the blend flow. They are there for a specific purpose.
Penetrating questions we are forced to answer
- If the formula worked in R & D, why does it not work now?
- Why does line management complain that they can barely achieve the minimum tablet hardness specified on the batch record?
- Why do some batches of the same product always seem to pick, while others are fine? Did they use the right excipients?
Let’s go back to basics
When a scientist discovers a new compound that helps a human, they initially have no idea what ultimate form that compound will take: injectable, aerosol, cream, liquid, capsule (soft or hard), or tablet.
Once a decision is made that this compound will be a tablet, we are entering into the world of powder and compaction science. So we either rely on the knowledge and skill of the R & D department and we suffer if the final formula has problems.
And if we have problems after the formula is submitted we sometimes ask the raw material supplier to provide an answer.
Excipients are for a specific purpose
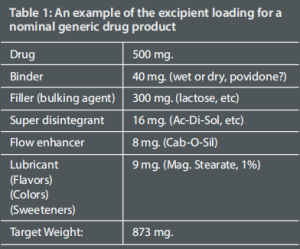
Different excipients have different functions. They are included for a specific reason, such as improving tablet disintegration. And while they accomplish their task they may add to the flow problem because of their particle level size and shape. Table 1 lists several excipients and their corresponding roles.



Granulation
Optimized powder flow is achieved when a tablet press can produce tablets at a high speed and with excellent content uniformity of the drug in the tablets.
Better powder flow is often achieved by gluing the various powders together in a process called granulation. When subsequently blended with “Slippery” powders, a granulated powder blend will often exhibit sufficient flow so as to be able to be compressed on a tablet press at high speeds.
Again, there are many granulating technologies that may be used for gluing powders together. In generic pharmaceuticals the most commonly used processes are called “High Energy (Shear)” granulating and “Fluidized Bed” granulating. Both glue the various powders together but the resulting granules may both flow differently and compress at very different speeds.
Even with a granulation process, flow is not always adequate:and most excipients in tablets are not there to improve powder flow. There is considerable overlap between excipients in common usage, since a given excipient is often classified differently by different practitioners in the field; or is commonly used for any of several different functions.
flow is not always adequate:and most excipients in tablets are not there to improve powder flow. There is considerable overlap between excipients in common usage, since a given excipient is often classified differently by different practitioners in the field; or is commonly used for any of several different functions.
So get to the point!
It is my experience that many formulas will compress well: If powder blends are stored correctly and compressed at the proper time, and if the tablet press is operated correctly.
formulas will compress well: If powder blends are stored correctly and compressed at the proper time, and if the tablet press is operated correctly.
The operators, IPQA and management alike have an obligation to optimize and constantly challenge their thinking about critical operating machine set points that affect powder flow, as well as other individual product attributes.
The generally accepted Critical Control Parameters (CCPs) for a tablet press (and many encapsulators) include two control settings that many companies overlook; even in the face of published research, Form 483s/warning letters citing these very settings.
Let us review them now. For these settings and practices in part account for why we have poor powder flow and yet incorrectly blame excipients or formulas for the problem.
All powder blends have an optimum compressing window
 When we blend powder, we trap air. Air usually (but not always) helps the powder flow. Compress the batch while the air remains and you have sufficient powder flow. (But you have to take care as there is a capping danger!)
When we blend powder, we trap air. Air usually (but not always) helps the powder flow. Compress the batch while the air remains and you have sufficient powder flow. (But you have to take care as there is a capping danger!)
But wait too long and the air will leave. And even a perfect formula will not change this outcome. And once the powder settles there is no assurance that the powder will ever flow again.
Critical Process Parameters (CPPs)
There are ten generally accepted critical process parameters for a modern tablet press:
- Fill volume: weight
– Compression force* (on most presses)
- AWC min/max weight limit settings
- Pre-compression thickness
- Main or final thickness
- Pre-compression punch entry
- Final compression punch entry
- Powder Hopper:
– height
– position of a slide gate or valve above the powder hopper
- Feeder speed
- Turret speed
- The punch protection (Overload) system
* Many companies do not view compressing force as a true CPP. Rather it is viewed as a product of the actual fill volume and thickness setting at a specific turret speed.
Common mistakes made by tablet press operators
Tablet presses are the most complicated, simple machines to misunderstand. Constant refresher training is required to maintain the skills necessary to operate a tablet press at the highest level. Tablet press operators should periodically review their practices to avoid making these common mistakes:
- Using the wrong fill (dosing) cam.
- Using only an Allen wrench or using the wrong torque value for setting the die lock screws.
- Using the wrong pressure for the lower punch retention pins.
- Using an incorrect setting for the feeder, the scraper or both.
- Allowing significant variation of powder levels in the press hopper.
- Leaving the slide gate/valve 100% open/Allowing too much powder in the feeder.
- Running the feeder speed too high.
- Setting the wrong tablet rejection limits/failing to verify at steady state.
- Failing to adjust ejection height.
- Failing to adjust the knock off plate (take off plate, tablet scraper) correctly.
- Using the wrong level of punch lubrication.
- Failing to verify the overload set point.
- Accepting any setting of the vacuum system.
Summary and Conclusion
- There is an optimum time to compress every powder blend.
- Optimized powder flow on a tablet press is a complicated task.
- The position of a valve or slide gate on a tablet press or encapsulator is a critical operating parameter (CPP).
- Powder feeders are often over filled and this can bring unnecessary weight variation and/or content uniformity problems.
- Flow issues resulting in both content uniformity and weight variation are very often operator and machine related problems.
Don’t blame the excipients!







