
By: Varadharaj Vijayakumar
This article describes the hold time expectation for the partially stoppered media filled vials during validation of aseptic process for lyophilized products. Aseptic process simulation, also known as a media fill trial, estimates the contamination risk of an aseptic production process by using sterile culture media in place of the product constituents.
Purpose of media fill
The goal of a media fill is to demonstrate that the manufacturer can follow the routine aseptic production process using sterile media without contamination (evaluate and justify the aseptic capabilities of the process, the people and the system).Hence in this article, we ensure lyo media fill should primarily validate the filling, transportation and loading and unloading aseptic operations with certain duration of filled vials in the lyophilizers.
BACKGROUND
During aseptic process simulation for lyophilized product, filled vials are aseptically filled in the normal way (similar to liquid filling), but the closures (which are of a single slotted or double slotted) are not fully inserted in the filled vials and transported aseptically through conveyor/ALUS/robotics to the lyophilizer shelves under Grade A atmosphere
Loading of media filled vials in the lyophilizer
Shelf to be pre cooled to 20-25°C. The media filled vials are loaded in the sterilized lyophilizer (using suitable in-house designs such as automatic loading/manual loading using fences). At the completion of the loading process, it is important to ensure all filled, partially stoppered vials are loaded into the lyophilization chamber.
Product lyophilization process
Lyophilization or freeze drying is a process in which water is removed from a product after it is frozen and placed under a vacuum, allowing the ice to change directly from solid to vapor without passing through a liquid phase.
What will happen if it’s simulated for longer hours of holding similar to product lyophilization?
Exact simulation of media fill as that of product lyophilization will have possible reduction of microbiological levels after aseptic manipulation which will not solve the purpose of aseptic process simulation. Carrying out a lyophilization cycle and freezing the media will be same simulation as that of product lyophilization, however this freezing of media will reduce microbial levels of some contaminants.
In aseptic process simulation of lyo process, the scope is not to check the lethality of freezing and its effect on microorganisms that might be present. Hence freezing of media and the formation of ice crystals is unfavorable to microorganisms, and this should be avoided.
Unfrozen media and complete vaccum
If media is not frozen, the lyophilzer may get contaminated due to unfrozen media which is left under vacuum similar to the product lyophilization vacuum level. If it’s complete vacuum drawn, it may cause the media solution to get out from the containers and contaminate the lyo as well as fluid loss from the containers, and finally this may have serious effect on the viability of the microorganism, and the ability of the media to support microbial growth will be impacted. This will in turn make aseptic process simulation invalid. Hence a complete vacuum as specified for the lyophilization process should not be drawn during the media fill.
Hold time duration in the lyophilizer
In general the course and processing time should be long enough to challenge or stress the process, interventions, the supporting environment, and the operators. All efforts should be made to perform all routine interventions. The filled partially stoppered vials will remain inside the lyophilizer chamber for minimum 12 hours (Author’s experience for hold time duration).The duration of the media filling stated above represents lyophilization cycle for one shift.
During this we ensure the integrity of the lyophilizer chamber by testing the lyophilizer post sterilization cycle. Therefore, no additional benefit is drawn by holding the media filled vials for longer duration (such as product lyophilization cycle) since the lyophilizer’s integrity is maintained thought out this cycle. Hence, it is not necessary to carry out lyophilization cycle as per the actual drug product lyophilization cycle. As compared to lyophilization hold duration for filled vials in lyophilizer, the chances of contamination for partial stoppered vials is more during the process stages such as empty vial transportation, media filling, samplings, half stoppering, loading of vials into lyophilizer etc.
Highlight
In general the course and processing time should be long enough to challenge or stress the process, interventions, the supporting environment, and the operators. All efforts should be made to perform all routine interventions. The filled partially stoppered vials will remain inside the lyophilizer chamber for minimum 12 hours.
So how long the APS process shall be simulated?
Typically 600 mbar to 700 mbar. A few manufacturers used maximum of 900 mbar of partial vacuum and held for about 12 hours, and a few others held it for two hours and then it was broken by sterile filtered compressed air, instead of the nitrogen. The full stoppering of vials shall be performed using the stoppering mechanism at ambient pressure, and open the door and unload the vials aseptically and transfer to sealing machine. All the activity shall be performed in class 100 (Grade A).
Possible questions from regulatory agencies for lyo media fills:
- Is the aseptic handling of lyophilized products validated by media fills?
- In the aseptic process, is lyophilization simulation performed during media fill?
- Is the maximum amount of time the vials are held prior to lyophilization simulated during media fills? If vials are not sealed in lyophilization chamber, is the maximum hold time prior to stoppering simulated in media fills?
- During validation, what level of vacuum is pulled on the lyophilization chamber?
- How long do media fill vials remain in the lyophilization chamber under vacuum? How does this compare to commercial lots?
- Does the process simulation result in freezing of the media? Note that this process simulation should not include freezing of the media.
- Is environmental monitoring performed during loading of the lyophilizer both during production and as well as during validation?
- Does the firm have data on growth promotion of the media? Are growth promotion tests done on vials after incubation is completed?
- Is environmental monitoring performed during unloading of the chamber during production as well as during media fill validation?
- What is used to break the vacuum during media fills (nitrogen, air, other gas)?
In my view, with practical experiences and conducted more than 30 lyo media fills:
Some Rationale
Vial and stopper size: The neck size of the vial is considered as a deciding parameter for the media fill. The other parameters like body diameter are considered as non deciding parameters for the media fill. The vials having neck diameters of 13 mm, 20 mm and 32 mm are generally used in industry. The size of the vial shall be considered while deciding the worst case for the media fill.
The size of the rubber stopper is deciding parameter of the media fill and hence is considered as a critical parameter. The rubber stoppers of size 13 mm, 20 mm and 32 mm are generally used in industry. The size of the rubber stopper shall be considered while deciding the worst cases for the media fill.
Type of vial and stoppers: The tubular or molded vials are in use. For the media fill, the tubular vials are considered as worst case since the tubular vials have lesser weight and larger movement or tendency to topple. The stoppers of the bromobutyl and chlorobutyl are generally used, and the type of the stopper is not a deciding parameter for the media fill and stopper of any type can be used for the media fill.
Source of vial: The source of vials is not a deciding parameter for the media fill and hence any source of vials can be used.
Finishing and colour of vials and stoppers: The finish of vial – blow back or non blow back and treated or non treated – does not play a deciding role in media fill and hence the vial of any finish can be used. The clear glass vials shall be used for the media fill to facilitate the visual inspection of the vials. The rubber stoppers coated and non-coated are used generally but not as deciding parameter for the media fill. But design of stoppers are important and plays a deciding factors, e.g. 2-leg, 3-leg igloo or flat-teflon, serum etc.
Fill volume: The volume of medium must be sufficient to provide contact with all container closure seal surface on inversion, and visibility for the detection of microbial growth. The quantity of sterile SCDM medium shall be considered for filling in the vial with different sizes are as follows: 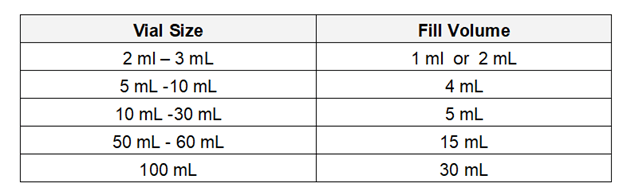
Pulling and releasing of partial vacuum: Pull the condenser of lyo to -40 °C and start the vacuum pumps. The loaded vials are exposed to the partial vacuum cycle.
First pull of vacuum: Pull the vacuum to 650 mbar. After achieving the vacuum of 650 mbar, maintain it for one hour and release the vacuum gradually using filtered (0.22 micron sterile filter) compressed air up to atmospheric pressure. The half stoppered vials will be held at atmospheric pressure for approx one hour.
Second pull of vacuum: Pull the vacuum to 650 mbar after about one hour of first pull of vacuum, and after achieving the vacuum of 650 mbar, maintain it for one hour and release the vacuum gradually using filtered (0.22 micron sterile filter) compressed air up to atmospheric pressure. The half stoppered vials will be held at atmospheric pressure for approx one hour. After this period perform the stoppering of vials into the chamber at atmospheric pressure. Note: In case of anaerobic media fill, break the vacuum of lyophilizer using filtered nitrogen gas, instead of filtered compressed air.
How many vials shall be loaded?
- Minimum 10000 vials shall be filled during media fill.
- Minimum number of vials to be filled shall be decided based on equipment constrain, where number of vials shall be filled considering the maximum capacity of the equipment.
Note: The media fill duration shall be sufficient to cover actual duration of the filling of the drug product. The leftover media after completion of the filling shall be measured and discarded.
Some FDA 483 observations for quick look….
Observation
The protocol stated that chamber for the lyophilizer must be held under slight vacuum conditions to simulate the process. The slight vacuum conditions were not created during the hold time when the media filled vials were in the lyophilizer chamber.
CONCLUSION
The duration of the media filling stated above represents overnight lyophilization cycle. We ensure the integrity of the lyophilizer chamber by testing the lyophilizer post SIP cycle. Therefore, no additional benefit is drawn by holding the filled vials for longer duration since the lyophilizer’s integrity is maintained throughout cycle. Hence, it is not necessary to carry out lyophilization cycle as per the actual drug product lyophilization cycle. As compared to lyophilization hold period, probability of contamination of vials is more during the process stages such as filling, stoppering etc. And also as per regulations from a few regulatory authorities, it’s clear that hold time does not need to be the actual duration of lyophillization cycle. However, there should be written justification for the hold duration of media fill vials in the aseptic simulation process of lyo process.
 Hence, a balanced risk and science based approach is needed to simulate the process as closely as possible, and rationale for holding the vials in the lyophilizer need to be presented, which is the expectation from the regulators. Finally, in case of failure in the media fill it necessary to diagnose and prove the source of contamination accurately so that robust corrective or preventive actions get implemented.
Hence, a balanced risk and science based approach is needed to simulate the process as closely as possible, and rationale for holding the vials in the lyophilizer need to be presented, which is the expectation from the regulators. Finally, in case of failure in the media fill it necessary to diagnose and prove the source of contamination accurately so that robust corrective or preventive actions get implemented.







