How to minimize heating formation for an ibuprofen blend
By F. Giatti, N. Pistillo and C. Funaro, F.S. Consoli
The challenge for tablet press producers is to design machines that can be at the same time cost/price effective for those customers producing standard product and highly customizable for special application. A change at design concept of these commodities machine is mandatory to be able to answer properly to these market requests.

If it is true that there exists a wide range of easy-to-manufacture products that permits to justify this expectation, there is a significant area of product’s recipe that are not optimized for industrial mass production, i.e. old formulation that have to satisfy current level of quality. There are also products with very restrictive physical properties that require specific customization on standard machine to be machinable, i.e. thermosensitive products.
This case study has the aim to verify the manufacturability of aIbuprofen-based formulation, a well-known thermosensitive product while ensuring high output on an industrial double-side rotary tablet press. The focus was the ability to reduce heating formation by the insertion of an air treatment unit (ATU) and process optimization.
Material & Methods
If it is true that there exists a wide range of easy-to-manufacture products that permits to justify this expectation, there is a significant area of product’s recipe that are not optimized for industrial mass production, i.e. old formulation that have to satisfy current level of quality. There are also products with very restrictive physical properties that require specific customization on standard machine to be machinable, i.e. thermosensitive products.
This case study has the aim to verify the manufacturability of aIbuprofen-based formulation, a well-known thermosensitive product while ensuring high output on an industrial double-side rotary tablet press. The focus was the ability to reduce heating formation by the insertion of an air treatment unit (ATU) and process optimization.

Results
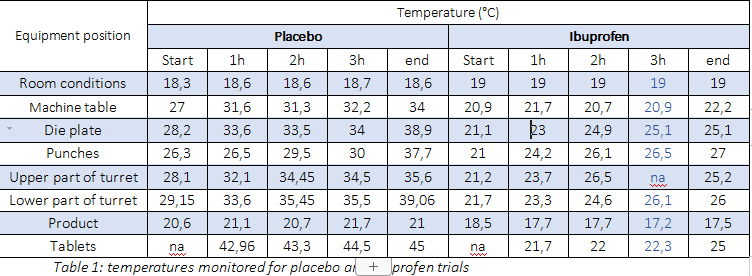
PREXIMA 800 rotated all night long in order to reach the mechanical inner overwarming as expected during a real production process of 3 shifts. Afterwards, the machine was producing for 4 hours tablets rotating at controlled ambient temperature condition of 19°. Temperatures were monitored in different equipment positions at the beginning, after 1 hour, after 2 hours, after 3 hours and at the end (almost 4 hours and 30 minutes) as reported in table 1.
The results were extremely interesting. The machine table that divides the mechanical area from the production area is the coolest part of the system, so that is possible to argue that the major part of the heat is not coming from the mechanical area. This result was expected because PREXIMA tablet presses use high efficiency gearbox and motor, as IMA technical choice.
Cooling systems are usually not part of a tablet press machine but are additional utilities that can be inserted in a standard layout.
The process area presents, on the top of the machine, inlet/outlet ports that can be used for an Air Treatment Unit, which controls the inlet temperature of the treated air in the process area, in order to obtain the required process temperature.The already proven solution allows to produce continuously tablets of an Ibuprofen-based formulation: target was to maintain always the temperature below 30 °C by maximizing machine output.
The final solution was using the 3 inlet point from the top in the process area and additionally also the lower inlet point. To cool down the process area at 8-20°C, the calculated flow rate was 1000m3/h at 14°C and relative humidity between 30-50%.
Repeating the same working condition of the preliminary placebo test to have comparative results, it was possible to reduce temperature (table 1).
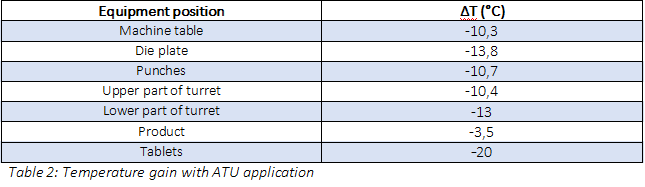
It is possible to extract the reduction (ΔT) between the temperature obtained during preliminary trials and with ibuprofen cooled-ATU application (table 2):
Die table and tablets, measured fully operational, have reached the same temperature, confirming that the main heat source is given by the tablet formation process.
The higher temperature achieved by the punches with respect to the other parts is mainly due to the friction during their movement. Heat generated during the production for friction is proportional to the rotation speed of the machine. Therefore, for a given fix speed, the temperature also increases up to a limited temperature, that remains under the 30°C.
Conclusion
The pro-active design of PREXIMA has permitted an easy implementation of an air treatment unit with no impact on the machine design and no additional cost for the customer. The costs were related directly to the ATU, confirming that flexibility is a design pillar that characterizes PREXIMA tablet presses and that has to be implemented in the machine concept. The payoff of such a strategy is not only the possibility to satisfy the customer’s need when required, but also to permit the implementation of a wide range of customization with cost related only to the additional optional required.
References
- https://www.pharmaworldmagazine.com/global-oral-solid-dosage-pharma-formulation-market-projections/
- https://drug-dev.com/growth-foreseen-at-all-levels-of-the-oral-solid-dosage-form-excipients-market/
- Technology transfer and process optimization for a ibuprofen based formulation,“Process Worldwide”, November 2020.

Federica Giatti
Compression Technologist at IMA Active
Graduated in chemistry and pharmaceutical technology cum laude, she specialized in solid dose processes from the beginning of her career. She started with coating during her thesis and improved her knowledge working in a pharmaceutical company. She is expert in tableting process development, troubleshooting and customer assistance.

Nicola Pistillo
Senior R&D Mechanical Engineer at IMA Active
He is Senior R&D Mechanical Engineer for tablet press machines. Graduated in Mechanical Engineering at the Polytechnic of Bari (Italy), Master in Business Administration at the Fachhochschule of Management in Cologne, he has been working in the design and development of tableting machines for almost ten years.

Caterina Funaro
Process R&D Laboratory Manager at IMA Active
Caterina Funaro got her degree in Chemistry and Pharmaceutical Technology at the University of Bologna in 1998. She is actually employed at IMA Active Division as process laboratory manager and her main responsibilities are technical assistance to sales for all solid dose equipment, after sales process assistance, R&D, training and cleaning support for solid dose manufacturing equipment.

Fabrizio Consoli
Technical Department Manager for Tablet Presses
In charge of the technical department for tablet presses, he manages both the R&D and the production machines for this product line. He joined IMA Active in 1995, working in the R&D department and developing transversal skills on the various automatic machines for the production of pharmaceuticals in oral solid dosage form.
IMA Exclusive
An exhaustive coverage of the world leader in the design and manufacture of automatic machines for the processing and packaging of pharmaceutical products. Wide portfolio of machines and the ability to offer tailor-made solutions to satisfy the most sophisticated requests in the market are the biggest strengths of IMA.









