
By Dr. Tim Sandle
An important objective of hygienic design relating to the pharmaceutical and healthcare sectors is with the avoidance of product contamination by microorganisms, particles and chemicals (1). When considering cleaning validation, microbial and chemical deposits and their removal require different approaches and they are influenced by different factors (something that needs to be accounted for in cleaning validation studies). However, the characteristics of surface design are described in general terms in this article.
To achieve the required hygienic state requires the application of good hygiene design techniques that allow for all assets to be cleaned effectively and efficiently in order to minimize the risk posed by these different hazards. One important consideration is with surface composition, in relation to surface smoothness and the density required to provide insulation.
In considering how these hygienic design factors are to be assessed, this article is concerned with specification, calculations and values that need to be applied to assess surface suitability. The objective is to provide information that can be factored into the design stage.
These factors are important because the physical properties of a surface, like charge and roughness, play a role in the adhesion of organic molecules. If non-ideal, this will cause challenges with cleaning validation. In addition, increased surface roughness will promote initial biofilm development and hence microbial colonization due to the larger surface area.
Surface roughness
The materials used for the construction of a pharmaceutical processing plant and equipment must fulfill certain specific requirements. Product-contact materials must be inert to the product under operating conditions, as well as to detergents and antimicrobial chemicals (disinfectants) under conditions of use. Materials selected must be corrosion-resistant, inert, mechanically stable, and such that the original surface finish is unaffected under all conditions of use. Furthermore, materials need to be non-toxic and non-absorbent. In addition, non-contact materials are also required to be mechanically stable, smoothly finished and easily cleaned. Where the reinforcement of equipment with plastics and elastomers is required, these should not be allowed to contact the product. Incompatible materials in the construction greatly increase the likelihood of product safety risks, for example through corrosion or pitting.
detergents and antimicrobial chemicals (disinfectants) under conditions of use. Materials selected must be corrosion-resistant, inert, mechanically stable, and such that the original surface finish is unaffected under all conditions of use. Furthermore, materials need to be non-toxic and non-absorbent. In addition, non-contact materials are also required to be mechanically stable, smoothly finished and easily cleaned. Where the reinforcement of equipment with plastics and elastomers is required, these should not be allowed to contact the product. Incompatible materials in the construction greatly increase the likelihood of product safety risks, for example through corrosion or pitting.
With reference to ‘smooth’ surfaces, this is something that directly affects ‘cleanability’. Hence, product contact surfaces need to be smooth enough to be easily cleanable. There are several different ways to assess surface roughness; however, the Ra value is the most commonly applied measure (‘Ra’ = roughness average). Thus, the Ra value expresses the roughness (or smoothness) of a surface, which is typically expressed in µm (the calculated Ra value is a dimensional unit). This value is quantified by the deviations in the direction of the normal vector of a real surface from its ideal form based on measurements taken using a device called a profilometer. With the instrument, a stylus is used on a material and the sound created is assessed and compared against a standard value to provide the Ra value for the material (2).
An assessment of the Ra value for different items of equipment is an important consideration since a high Ra increases the possibility of microbial attachment or chemical residues remaining affixed to the surface (especially where there is poor fluid flow, since the microbial colonization on such surfaces is preceded by the formation of a pellicle (a membrane formed by the presence of a fluid). The physicochemical surface properties of a pellicle are largely dependent on the physical and chemical nature of the underlying surface. There is also a time-bound efficiency consideration with the Ra value concept in that, generally, the cleaning time required increases with surface roughness. Furthermore, Ra values can increase as an item of equipment ages, so the Ra value at the time of the Factory Acceptance Test (FAT) may differ by the time an item of equipment has been used for five years and with a period of regular use (3).
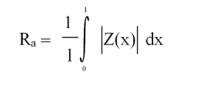
The commonly adopted ‘rule’ is that contact surfaces should have a maximum roughness of Ra = 0.8 µm (often the Ra requirement is <0.4μm). The standard for assessing surface roughness is ISO 4287:1997
Ra values are calculated as the arithmetical mean of the absolute values of the profile deviations (expressed as Z i) from the mean line of the roughness profile values. This is derived from the following calculation (4):
Where:
Z(x) = profile ordinates of roughness profile. This is the greatest height of the roughness profile calculated from the sum of the height of the highest profile peak and the depth of the deepest profile valley, relative to the mean line, within a sampling length lr i.
For critical equipment, the expectation is typically for an Ra-value between 0.2 and 0.5 µm. For ancillary equipment, such as CIP skids, steam generators and chiller higher Ra values can be used, with justification. The calculated mean Ra value is measured based on an assessment of five sampling lengths. Hence care must be taken when interpreting some Ra values. This is because the use of the Ra number reduces a series of measurements into a single number, and this number itself reflects the mean of the overall material being assessed. According to Schuetz (5), “Ra is calculated by an algorithm that measures the average length between the peaks and valleys and the deviation from the mean line on the entire surface within the sampling length. Ra averages all peaks and valleys of the roughness profile and then neutralizes the few outlying points so that the extreme points have no significant impact on the final results.” The accuracy of Ra values has latterly been improved through the use of digital measuring equipment.

To achieve the required quality of surface finish, this is either natural to the material or, more likely, the material is rendered more acceptable by electropolishing (an electrolytic process designed to remove metal without smearing or folding) or alternative surface treatments are put in place. With electropolishing, rough peaks are targeted for removal, resulting in a micro-smooth, reflective finish. Electropolishing has an added benefit in relation to reducing the likelihood of microbial adhesion. The adhesion of bacteria to a surface depends on a number of microbiological, physical, chemical, and material-related parameters, including topography. With electropolishing, the process modifies surfaces to limit the adhesion of microorganisms.
Stainless-steel is also commonly passivated (an-electrolytic finishing process that makes stainless steel more rust-resistant) (6). Passivation is normally undertaken with acid (often nitric or citric acid to remove free iron from the surface, which leads to an inert, protective oxide layer that is less likely to chemically react with air and cause corrosion). An alternative to stainless steel in anodized aluminum, where aluminum alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved adhesion (7). Ra is an important factor with stainless steel and cold-rolled stainless-steel sheet material is commonly used for vessels and for piping in the pharmaceutical sector.
Passivation is normally undertaken with acid (often nitric or citric acid to remove free iron from the surface, which leads to an inert, protective oxide layer that is less likely to chemically react with air and cause corrosion). An alternative to stainless steel in anodized aluminum, where aluminum alloys are anodized to increase corrosion resistance and to allow dyeing (coloring), improved lubrication, or improved adhesion (7). Ra is an important factor with stainless steel and cold-rolled stainless-steel sheet material is commonly used for vessels and for piping in the pharmaceutical sector.

A further consideration with stainless steel is its grade, with 316L being preferred over 304 (using SAE steel grade categorization, where 316L is equivalent EN 10027 Part 2: 1.4404 /1.4435 (8)); and this grade difference is essential when it comes to product contact parts. The grade of stainless steel is a reference to the different resistances to corrosion, strength, formability or welding properties (9). For critical processes and special applications, materials should be furnished with an inspection certificate under traceability procedures.
Ra can also be expressed as Rq, which is the root mean square of the roughness (Rq). This is based on calculating the root mean square average of the roughness profile ordinates. Other ways to express surface roughness are (10):
- The Single Roughness Depth (Rzi), which is the vertical distance between the highest peak and the deepest valley within a sampling length.
- The Mean Roughness Depth (Rz), which is the arithmetic mean value of the single roughness depths of consecutive sampling lengths. Rz is calculated by measuring the vertical distance from the highest peak to the lowest valley within five sampling lengths, then averaging these distances. Rz averages only the five highest peaks and the five deepest valleys – therefore, extremes have a much greater influence on the final value.
- The Maximum Roughness Depth (Rmax), which is the largest single roughness depth within the evaluation length. This tends to be used for surfaces in which individual deviations have a significant influence on the function of the surface, e.g. sealing surfaces. All roughness parameters with the suffix “max” represent the maximum mean value measured within five sampling lengths.
However, for simplicity and when evaluating pharmaceutical processing equipment, the use of the Ra value is the most straightforward and typically provides a point of common understanding between purchasers (pharmaceutical companies) and suppliers.
For the use of symbols for surface roughness in technical drawing, the reader can refer to standard ISO/CD 21920-1. For an indication of requirements for surface imperfections (pores, scratches etc.), which cannot be specified using surface texture parameters, this detail is provided in ISO 8785. This international standard covers the range of surface imperfections (however, the acceptance of any imperfections should be treated very cautiously within the pharmaceutical sector and generally imperfections should not be accepted).
A further factor for consideration with the finish of materials like stainless steel is the reaction to chemical used for cleaning or disinfection. A study into pitting corrosion resistance was evaluated in a commercial disinfectant and 1M NaCl. It was found that electropolished and grit 4000-polished steel proved more corrosion resistant as opposed to grit 80- and 120- polished surfaces.
Insulation
An R-value is additionally used to assess insulation, based on material thickens. Consideration of insulation is important for vessels which are heated, and ill-defined requirements or viability with insulation can lead to issues of concern in relation to cleaning. Furthermore, although it is outside the scope of this article, but of relevance to pharmaceuticals, it is worth noting that insulation is important for Heating, Ventilation and Air Conditioning (HVAC) system design and for controlling temperature when shipping product as part of supply chain management.

With equipment and in relation to cleaning processes, insulation is a measurement of a material’s capacity to resist heat flow from one side to the other (or the temperature difference that will cause one unit of heat to pass through one unit of area over a period of time). R-values measure the effectiveness of insulation and a higher number represents more effective insulation. When two or more items of equipment are required to connect, then R-values are additive, which means if there is a material with an R-value of 12 attached to another material with an R-value of 3, then both materials combined have an R-value of 15 (11). Unlike Ra values, the R-value for insulation is a dimensionless number.
R-values for insulation are expressed as:
R value = thickness (meters)/Thermal Conductivity (Watts / meters x temperature)
Or as shown in the following equation:
Where:![]()
- Rval is the R-value,
- Δ T is the temperature difference (expressed in Kelvins) between the warmer surface and colder surface of a barrier,
- ϕ q (as Watts per cubic meter) is the heat flux through the barrier.
R-values can also be used to assess U-values. The U-value is the heat transfer coefficient, which is a measure of an assembly’s capacity to transfer thermal energy across its thickness. The U-factor of an assembly is the reciprocal of the total R-value of the assembly. This is expressed as: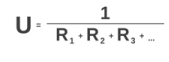
A U-Value is essentially a measure of heat loss through a structural element. This value is calculated on the rate at which heat transfers through 1 square meter of a structure, where the temperature difference between the inner and outer face is 1 degree Celsius.
With the assessment of insulation, it is important to note that R-values, similar to surface roughness measures, often deteriorate over time.
Surface crevices
To be cleaned without difficulty, surfaces must not only be smooth but also free from crevices, sharp corners, protrusions and shadow zones. This applies not only when equipment is new, but during its entire functional lifetime. With crevices, these need to be avoided because they cannot always be effectively cleaned, and hence crevices can retain the product residues that could cross-contaminate the next product to be processed or effectively protect microorganisms against inactivation from any disinfectant used or from heat. For lower-risk items of equipment, crevices might be unavoidable (such as the bottom bearings of top-driven stirrers or as bearings in scraped-surface heat exchangers). In such cases the presence of slide bearings needs to be considered when writing procedures for cleaning and disinfection. For deep cleaning there may need to be both the partial or total dismantling of equipment, or for increased cleaning times. It also stands that the use of screw threads and bolts in the product area should be avoided; furthermore, sharp corners in the product area should be avoided. Where crevices are present the radii should be >3mm or more. Furthermore, the typical corner within an item of equipment should be >90o, in order to achieve good cleanability.

The use of plastics, elastomers and lubricants are often unavoidable in relation to equipment. With plastics, these should be non-reactive capable of being disinfected with ozone or with other chemicals (since heating above a given level is not effective without damaging the plastic, as with polyvinylidene fluoride or polyvinylidene difluoride). Some plastics cannot be heated at all, such as Acrylonitrile Butadiene Styrene (ABS). Typically, plastics like acrylic and high-density polyethylene are used for fluid handling applications. Elastomers (“rubbers”) that are used, for example with gaskets, sealing or hoses, will be composed of a couple of ingredients, like polymers, fillers, plasticisers, activators, antioxidants, accelerants and vulcanizing agents. The constituent materials need to be assessed for their suitability since the composition determines the physical and chemical properties of the elastomer.
Viton, as an example, is a rugged rubber which is ideally suited for applications requiring hard-wearing O-rings, gaskets and seals.
Lubricants are a further consideration when assessing materials. Lubricants that are used in manufacturing equipment and that can come into contact with materials need to be food grade (since ‘pharmaceutical grade’ does not exist as a category for selection; the grading system for lubricants is defined by ISO 21469: 2006). Lubricant selection should begin by considering the lubrication needs of the component or machine in terms of the load, speed, viscosity and application method. Desirable properties for lubricants include:
- Water resistance,
- Oxidation resistance,
- Low wear rate,
- Compatibility with construction materials,
- Good adsorption and adhesion on metal and plastics,
- Steam resistance.
In addition, the flashpoint of this oil needs to be suitable. An oil with too low a flashpoint can lead to mechanical breakdown. While many items of equipment will come with cut-off features (to meet safety requirements), if the oil goes too high then the equipment will ordinarily not return to operational use until the oil temperature drops by about 40°C and the sensor resets. Often the oil flashpoint temperature is set at >230°C.
These requirements should be considered when purchasing new equipment, along with other design parameters, such as accounting for the volumes, agitator types, heat transfer areas, etc. These parameters should be detailed in the equipment’s user requirements specifications (URS) (12).
Maintaining equipment
Once pharmaceutical equipment has been purchased, it is important to maintain the equipment in a good state. Given the degree of effort placed on surface roughness and the finish, as described above, damage to the surface must be avoided, especially in the form of scratches or chemical corrosion since these will create new opportunities for chemicals to become deposited or for microorganisms to adhere. For surface abrasion, the Ra value will be affected by the presence of scratches or porosities and hence the careful use of equipment and regular assessment (followed by repair if necessary) are required. With corrosion, rougher surfaces are more susceptible than smoother surfaces to localized forms of corrosion such as pitting and crevice corrosion, which provides another demonstration of the importance of surface smoothness (13).
Conclusion
As this article has indicated, improperly specified and designed surfaces for pharmaceutical processing equipment may affect the microbiological and chemical safety of the product and even result in physical contamination. It is a simple ‘rule of thumb’ that rough surfaces will aid the attachment of chemicals and promote microbial adhesion and maturation (14). The question posed in this article was ‘how rough?’, and for this an explanation has been provided.
The lesson to be drawn means paying careful attention to the composition of surface materials in terms of the thickness and smoothness, and designing and maintaining them in such a way so that cervices are minimized.
References
- Blanchard, J.A. and Signore, A.A. (1995) Cost-Effective cGMP Facilities, Pharm.Eng. 15(2), 44-54
- Doblhoff-Dier, O. And Bliem, R. (1999) Quality control and assurance from the development to the production of biopharmaceuticals, Trends in Biotechnology 17 (7):266-270
- Sandle, T. (2017) The Problem of Biofilms and Pharmaceutical Water Systems, American Pharmaceutical Review, 20 (7): http://www.americanpharmaceuticalreview.com/Featured-Articles/345440-The-Problem-of-Biofilms-and-Pharmaceutical-Water-Systems/
- Schuetz, G. (2006) Quality Gaging Tips (Modern Machine Shop Books), Hanser Publications, Cincinnati, U.S.
- Schuetz, G. (2002) Surface Texture From Ra to Rz, Modern Machine Shop. At: https://www.mmsonline.com/columns/surface-texture-from-ra-to-rz
- ISO 4287:1997 Geometrical Product Specifications (GPS) — Surface texture:Profile method — Terms, definitions and surface texture parameters, International Standards Organization, Geneva
- Chowdary, B.V. and George, D. (2012) Improvement of manufacturing operations at a pharmaceutical company, Journal of Manufacturing Technology Management, 23 (1): 56-75
- EN 10027-2:2015 Designation systems for steels. Numerical system, CEN-CENELEC, Brussels, Belgium
- Bringas, J. E. (2004). Handbook of Comparative World Steel Standards: Third Edition (3rd ed.). ASTM International. p. 14
- Ahn, D., Kweon, J. H., Kwon, S., Song, J., and Lee, S. (2009) Representation of Surface Roughness in Fused Deposition Modeling, J. Mater. Process. Technol., 209 (15): 5593–5600
- Kreider, J. F., Curtiss, P. S.; Rabl, A. (2010). Heating and Cooling of Buildings: Design for Efficiency (Revised Second ed.). Boca Raton, FL: CRC Press. p. 28
- Witcher MF (2017) Analyzing and managing biopharmaceutical risks by building a system risk structure (SRS) for modeling the flow of threats through a network of manufacturing processes. BioProcess J., 16. Available at: https://doi.org/10.12665/J16OA
- Hilbert, L., Bagge-Ravn, D., Kold, J., Gram, L.(2003) Influence of surface roughness of stainless steel on microbial adhesion and corrosion resistance,International Biodeterioration & Biodegradation, 52 (3): 175-185
- Busscher, H.J. and Weerkamp, A.H.(1987) Specific and Nonspecific Interactions in
Bacterial Adhesion to Solid Substrata, FEMS Microbiology Reviews, 46 (2): 165–173









