By Dr. Devang Patel, Dr. Rahul Haware, Kerry Cruz, Robert Sedlock
Tableting of the blended powder formulation with or without granulation is an important cog of a tablet manufacturing chain. This decision dictates a transformation of the blended powder formulation into a robust tablet.
The granulation step imparts the suitable powder bulkiness, flowability, and strength required for: an easy handling, a uniform dispensing from the hopper, and to confer the desired strength of the final tablet.
Two granulating methods such as dry granulation (roller compaction) and wet granulation are used in the pharmaceutical tableting process. Tableting without granulation is called Direct Compression (DC). A dry granulation roller compaction is used for the moisture and thermo-sensitive bulkier and poorly flowing materials [1].
A downside of dusty dry granulation is its inability to handle poorly compressible materials and impaired material re-workability. The wet granulation is executed for low and high dose formulations. The active pharmaceutical ingredient (API) binding with the excipients during wet granulation reduces the potential segregation of the low dose formulation. It can relegate elasticity concerns associated with the poorly compressible high dose formulations.
The main snare of such multi-step and labor-intensive granulation process is its unsuitability for the moisture- and thermo-sensitive APIs [2].
Non-granulating, cost-effective, DC methodology is a ‘process of choice’ in today’s high-paced modernized tablet manufacturing arena. The US FDA has provided the recommendations about continuous pharmaceutical processing under the ICHQ8 guideline. This is also called as ‘Process Analytical Technology (PAT).
A pharmaceutical continuous manufacturing is achieved with a real-time process monitoring using PAT tools. These PAT tools include various advanced spectroscopic near infra-red and Raman spectroscopy sensors. PAT sensors are placed inside the instruments for providing a real process monitoring to avoid any process deviations from the set standards or specifications. A DC process has fewer steps than other granulating process. This feature provides relatively easy adoption for a possible continuous manufacturing using PAT sensors.
Certainly, tableting with and without granulation have their unique advantages and limitations. The final formulation features are the main player, which prescribe the choice of a granulating and non-granulating methodology regardless of their advantages and inadequacies.
The main aim of the present study is to understand how the granulating and non-granulating methodology choices are transformed in the tableting performance depending on the API amount. More specifically, this study examines whether granulating and non-granulating choices are the functions of poorly compressible API amount in the final formulation. A model acetaminophen formulation was used to test this hypothesis.
Experimental Design
A poorly compressible acetaminophen formulation was selected in the fractions of 20% w/w, 40%w/w, and 60%w/w. PROSOLV® SMCC90 (silicified microcrystalline cellulose) was used as a binder. Its amount in the final formulation was varied depending on the amount of acetaminophen. Magnesium stearate (0.5% w/w) was employed as a lubricant and glidant. A total of three acetaminophen formulations were generated.
Acetaminophen formulations were dry granulated and wet-granulated with Alexanderwerk WP120 Roller Compactor and Diosna Laboratory Mixer-Granulator, respectively. The generated dry and wet acetaminophen granules,as well as acetaminophen formulations prepared using non-granulating direct compression method, were compacted using Natoli NP-RD30 4 stations rotary tablet press at 50, 100, 150, 200, 250, 300 MPa compression pressures and 25 RPM turret speed.
An important component of USP<1062> such as tabletability profile (tensile strength vs. compression pressure) was used to compare the impact of granulation and non-granulation method on the tablet robustness of the model acetaminophen formulations.Strain rate sensitivity study was also performed at 150 MPa compression pressure and 20, 40, 60, 80, 100 RPM turret speed to study the impact of granulating (wet and dry) and non-granulating methods on the formulation sensitivity to the applied compression speed.
Key Findings of the Study,
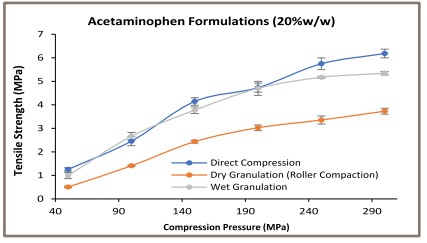
The acetaminophen formulations tabletability profiles are shown in Figure 1. Tablets of directly compressed and wet granulated formulations containing 20% w/w and 40% w/w acetaminophen exhibited similar tabletability profiles. These profiles are different from the dry granulated acetaminophen formulations.
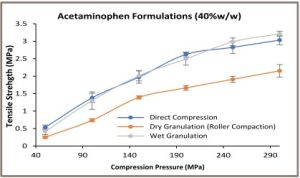 The dry granulated tablets of 20% and 40% w/w acetaminophen showed lower tensile strength at a given compression pressure when compared to the directly compressed and the wet granulated acetaminophen formulations. A weaker tableting of the dry granulated acetaminophen formulation could be attributed to the impaired material reworkability during the dry granulation [4].
The dry granulated tablets of 20% and 40% w/w acetaminophen showed lower tensile strength at a given compression pressure when compared to the directly compressed and the wet granulated acetaminophen formulations. A weaker tableting of the dry granulated acetaminophen formulation could be attributed to the impaired material reworkability during the dry granulation [4].
The compact sheets formed in the roller compaction step of dry granulation utilize the material’s deformation potential. These sheets further experience milling stress in their transformation into dry granules of the desired size. Such multi-step dry granulation could consume a substantial amount of the material deformation potential. Consequently, engineered dry granules display a significant reduction in the material deformation during the actual compression cycle phase of the tableting. It leads to the formation of weaker tablets, which is called a ‘reworkability issue’ of dry granules [4].
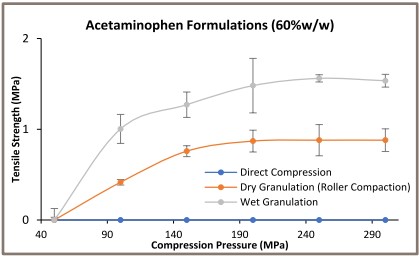
Figure 1: Tabletability Profiles
The tabletability profile trend was changed by increasing the acetaminophen amount in the formulation (> 40% w/w). The wet granulated formulation containing 60%w/w acetaminophen formed the stronger tablets as compared to the dry granulated formulation.
Acetaminophen is known as a poorly deforming elastic material [5]. These findings indicate the wet granulation is a ‘right choice’ of handling elasticity issue of such poorly compressible high-load formulation, regardless of its above-mentioned limitations.The inability of direct compression methodology to form tablets with 60% w/w acetaminophen formulation appears to confirm the study’s hypothesis.
Figure 2: Strain Rate Study (Tensile Strength Vs. Tangential Velocity)
In the second stage, the compression speed sensitivity of the acetaminophen formulations was analyzed by plotting the tablet tensile strength as a function of the tangential velocity (Figure 2). The turret speed of 20, 40, 60, 80, 100 RPM represents the tangential velocity of 209.3, 418.7, 628.0, 837.3, 1,046.7 mm/s respectively, based on 200mm Pitch Circle Diameter (PCD) of Natoli NP-RD30 Tablet Press. The maximum commercial rotary tablet speed is 90 RPM for an 840 mm diameter turret. It translates into approximately 3,958 mm/s tangential velocity. The applied tangential velocity in the present study was 200 mm/s to 1,100 mm/s.
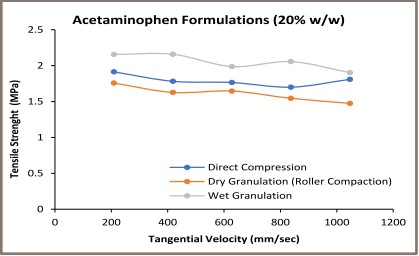
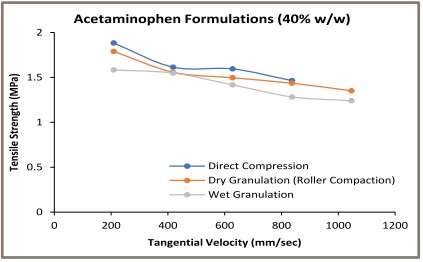
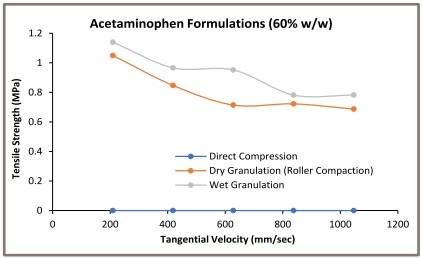
Figure 2: Strain Rate Study (Tensile Strength Vs. Tangential Velocity)
A material deformation is a time dependent phenomenon. A slow compression speed provides a sufficient time for the translation of an applied compression stress on the material during the compression cycle [6]. It led to a better material deformation necessary for a particle bonding during the compact formation. The compression speed is an important issue specifically with the deforming materials as compared to the fragmenting materials. Deforming materials produce weaker tablets at high compression speed due to an impaired deformation.
The wet granulated formulations containing 20% and 60%w/w acetaminophen exhibited high tablet tensile strength at applied compression speed as compared to the dry granulated and directly compressed formulations. The similar pattern was not found with 40%w/w acetaminophen formulation. Acetaminophen is elastically deforming material [5]. Overall, the wet granulated poorly compressible acetaminophen formulation exhibited less sensitivity to the compression speed as compared to the dry granulated and the direct compressed acetaminophen formulations.
Take Home Message
The wet granulated acetaminophen formulation produced stronger tablets. These formulations exhibited less sensitivity to the applied compression stress. Thus, the wet granulation performed using the Diosna Laboratory Mixer-Granulator was a better choice when compared to the direct compression or the dry granulation for acetaminophen formulation. This is specifically important when the amount of poorly compressible elastic acetaminophen exceeded 40%w/w in the final formulation.
References:
- Parikh, D. M. (2016). Handbook of Pharmaceutical Granulation Technology, Third Edition. CRC Press.
- Arndt, O. R., Baggio, R., Adam, A. K., Harting, J., Franceschinis, E., &Kleinebudde, P. (2018). Impact of Different Dry and Wet Granulation Techniques on Granule and Tablet Properties: A Comparative Study. Journal of Pharmaceutical Sciences, 107(12), 3143–3152. https://doi.org/10.1016/j.xphs.2018.09.006.
- Center for Drug Evaluation and Research. (2020, April 14). Q8(R2) Pharmaceutical Development. U.S. Food and Drug Administration. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q8r2-pharmaceutical-development.
- Sun, C. C., &Himmelspach, M. W. (2006). Reduced tabletability of roller compacted granules as a result of granule size enlargement. Journal of Pharmaceutical Sciences, 95(1), 200–206. https://doi.org/10.1002/jps.20531.
- Akande, O., Rubinstein, M., Rowe, P., & Ford, J. (1997). Effect of compression speeds on the compaction properties of a 1:1 paracetamol–microcrystalline cellulose mixture prepared by single compression and by combinations of pre-compression and main-compression. International Journal of Pharmaceutics, 157(2), 127–136. https://doi.org/10.1016/s0378-5173(97)00185-3.
- Haware, R. V., Tho, I., & Bauer-Brandl, A. (2009). Application of multivariate methods to compression behavior evaluation of directly compressible materials. European Journal of Pharmaceutics and Biopharmaceutics, 72(1), 148–155. https://doi.org/10.1016/j.ejpb.2008.11.008.
ABOUT THE AUTHORS
Dr. Rahul Haware is a Pharmaceutical Scientist currently serving as the Director of Scientific Support and New Business Development at Natoli Scientific. His professional and academic expertise is in leveraging Process Analytical Technology (PAT), and Quality-by-Design (QbD) paradigms to develop the DM(3) approach. He was a professor for eight years and has authored several book chapters (3) and peer-reviewed international review and research papers (46). Dr. Haware holds Master’s degrees in Pharmacy and Molecular Biology. He completed his PhD in Powder Compaction Physics at UiT The Arctic University of Norway.
Dr. Devang Patel is a Senior Research Scientist at Natoli Scientific. He uses his experience in resolving or providing guidance on various tableting issues like sticking, picking, etc. to provide scientific and technical support for Natoli’s customers by generating application data for formulation and tablet tooling. Some of his notable academic contributions include peer-reviewed papers on formulation and 3D printing. He holds a PhD and Master’s degree in Pharmaceutical Science from Long Island University, New York.
Robert Sedlock is the Director of Natoli Scientific. He is a leading expert in the tablet compression industry with over 25 years of experience and has authored numerous technical articles and journal publications. Mr. Sedlock is responsible for global solid dosage customer support, training seminars, contract compression services, and continuous research.
Kerry Cruz works as an Office Administrator and Lab Technician at Natoli Scientific, which is an Industrial Machinery & Equipment company. She is an integral part of the Office Operations team within the Operations Department.